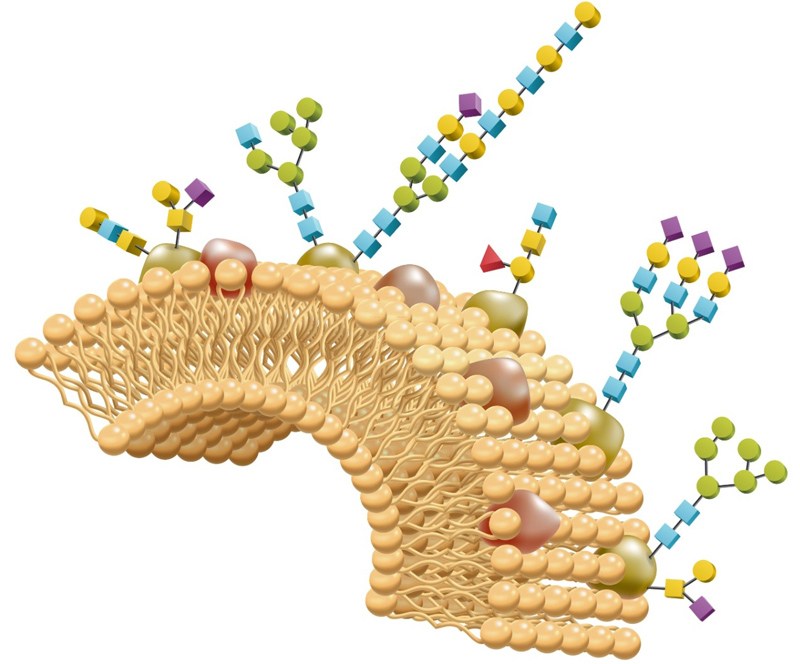
Glycomics, the study of glycans, is applied to biology and chemistry that focuses on the structure and function of carbohydrates, and on glycoform distributions at the cellular, tissue, organ and organism levels. In glycomics, there are three major parts, including structural characterization of glycans, research of glycan-protein interactions, and the study of in vitro and in vivo systems for the determination of the function of specific glycans. According to the backbone chemical structure, glycans can be divided into linear and branched sugars. The majority of the linear sugars are glycosaminoglycans (GAGs) that consist of repeating disaccharide units. The units are O-linked to a core protein, forming a proteoglycan aggregate. Branched glycans are present as N-linked and O-linked glycosylation on glycoproteins or on glycolipids. In mammalian cells, major classes of glycans include glycoproteins, glycosphingolipids, and proteoglycans. Glycoproteins are glycoconjugates in which diverse glycans are conjugated to a serine/threonine (O-linked glycoproteins) or an asparagine residue (N-linked glycoproteins) of the polypeptide backbone. Glycosphingolipids contain mono- or oligosaccharides that are linked to ceramides. Proteoglycans are glycoconjugates in which glycosaminoglycans are attached to a serine/threonine residue of the polypeptide backbone via a xylose moiety.
Figure 1. Major classes of glycans in mammalian cells (Hyun J Y. et al., 2017)
Speaking of the general analysis strategies for glycomics, glycoproteins/proteoglycans or glycolipids can release free glycans by enzymatic or chemical methods. The glycans can be derivatized, separated, or further analyzed by mass spectrometry (MS). Or the glycan can also directly be analyzed by MS after derivatization. In the shotgun glycomics for functional studies, the glycan microarrays are generated for analysis. For site-specific glycosylation and identification of protein carriers, glycopeptides can be generated by proteolysis and then analyzed directly before or after glycan removal.
Figure 2. General glycomics strategies (Cummings R D and Pierce J M, 2014)
1. Sample preparation. Because of the low abundance of glycoproteins, the glycoproteome or glycome must be pre-separated before mass spectrometry (MS) analysis. There are different enrichment strategies on glycoproteomes or glycomes. For example, the glycoproteome can be selectively enriched through lectin enrichment, hydrophilic liquid interaction chromatography (HILIC), boronic chemistry, hydrazide chemistry (HC), reductive amination chemistry, oxime click chemistry, or non-reductive amination chemistry.
2. Glycan release. The release of glycans from the glycoprotein is always the first step for analysis. There are two general methods, enzymatic and chemical methods. For enzymatic methods, specific enzymes are utilized to release N-linked glycans from glycoproteins, including two types of enzymes, endoglycosidases, and exoglycosidases. The endoglycosidases release most of the glycan, while the exoglycosidases remove only a very specific portion of the non-reducing end of the glycan. Common endoglycosidases are the PNGase (protein N-glycosidase) family which are amidases releasing most N-glycans. For example, PNGase F can cleave the bone between the asparagine residues of glycoproteins and the innermost GlcNAc, which release the carbohydrate and preserving the intact glycosylamine at the reducing end, although the amino group is quickly hydrolyzed and replaced by a hydroxyl group. Chemical methods include alkaline elimination and hydrazinolysis, which can preserve the carbohydrate moiety but degrades the glycoprotein. It is often used to release O-linked glycans from glycoproteins.
3. Derivatization of glycans. The poor ionization of native glycans or glycopeptides can hinder the MS analysis, so several chemical derivatization strategies with different labels have been developed. After derivatization, the ionization efficiency of glycans and glycopeptides in MS can be improved through increasing hydrophobicity. In addition, derivatization can also influence fragmentation patterns to facilitate structural analysis. There are various techniques available for glycomic and glycoproteomic derivatization, including reductive amination, hydrazide labeling, permethylation, and amidation.
4. Separation of glycans. This step is not always needed, but it can help to reduce sample complexity. In most cases, a wide range of isomeric or closely related structures of carbohydrate moiety needs high performance separation techniques before mass spectrometry. Qualitative and quantitative analysis of native glycans by the combination of high-performance liquid chromatography (HPLC) and MS can remarkably reducing efforts and time requirements. LC-MS can be used to detect glycans/glycoconjugates with low abundance, including N-linked glycans, O-linked glycans, glycopeptides, and glycolipids, and it also permits for the analysis of complex mixtures. There are some other methods available, including porous graphitized carbon chromatography (PGC), hydrophobic interaction liquid chromatography (HILIC), ion mobility-mass spectrometry (IM-MS), traveling wave ion mobility spectrometry (TWIMS).
5. MS analysis. MS can separate and detect ions according to their mass-to-charge (m/z) ratios. Nowadays, matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) are the two main MS ionization techniques for the analysis of carbohydrates. Collision-induced dissociation (CID), which means ions are fragmented by interacting with neutral collision gases (e.g. nitrogen or argon), is the most commonly used ion dissociation method in glycoproteomics and glycomics. Especially N-linked glycoproteomics, much attention has been paid to CID for analysis of deglycosylated peptides or glycopeptides. Higher-energy collisional dissociation (HCD), Electron capture dissociation (ECD), electron detachment dissociation (EDD) and Electron transfer dissociation (ETD) have been used in glycomics analysis.
MS is widely used in free oligosaccharides, glycosaminoglycans as well as the glycan portions of glycoproteins, proteoglycans, and glycolipids. And there is a wide range of MS techniques are available for glycomics analysis. As research continues on MS-based glycomics analysis, we believe that clear understanding the mechanisms of important biological processes will be achieved in the near future.
Equipped with state-of-the-art facilities, Creative Proteomics can provide reliable glycomics services in an affordable manner. Our team of experts with extensive experience can help you understand what you are trying to investigate and give you the most appropriate solutions. Our glycomics services include:
- N-Glycan Profiling
- O-Glycan Profiling
- N-Glycosylation Site Occupation Analysis
- O-Glycosylation Site Occupation Analysis
- N-Glycan Linkage Analysis
- O-Glycan Linkage Analysis
- Structural Characterization of Glycans
- Glycopeptides Analysis
- Glycans-related Microarray Assay
- Polysaccharide Analysis
- Peptidoglycan Structure Analysis
References:
- Lu H, Zhang Y, Yang P. Advancements in mass spectrometry-based glycoproteomics and glycomics. National Science Review, 2016, 3(3): 345-364.
- Han L, Costello C E. Mass spectrometry of glycans. Biochemistry (Moscow), 2013, 78(7): 710-720.
- Hyun J Y, Pai J, Shin I. The Glycan Microarray Story from Construction to Applications. Accounts of chemical research, 2017, 50(4): 1069-1078.
- Cummings R D, Pierce J M. The challenge and promise of glycomics. Chemistry & biology, 2014, 21(1): 1-15.