Encapsulation Efficiency (LNP) Determination of DNA & RNA Drugs
At Creative Proteomics, we acknowledge the paramount importance of effective drug delivery in ensuring the efficacy and safety of your DNA & RNA therapeutics. Our encapsulation efficiency (VLP) determination services provide a comprehensive evaluation of drug formulations, guaranteeing maximum drug encapsulation and improved therapeutic outcomes.
Introduction of Encapsulation Efficiency (LNP) Determination
The determination of encapsulation efficiency (LNP) entails the quantification of DNA & RNA drugs enclosed within lipid nanoparticles (LNPs). LNPs are nanoparticles composed of lipids that can encapsulate and shield therapeutic molecules, such as oligonucleotides, enabling efficient delivery to target cells. Encapsulation efficiency (EE) is expressed as a percentage and signifies the proportion of DNA & RNA drugs effectively integrated into the LNPs concerning the total amount of drug employed during the formulation process. A high EE value is desirable, indicating that a substantial portion of the drug is safeguarded within the LNPs, enhancing its stability and potential for successful delivery.
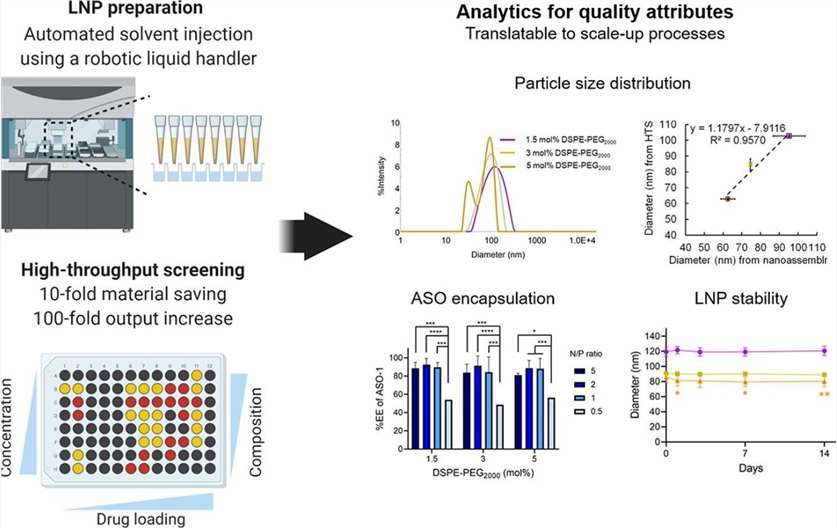 Fig. 1. Preparation and characterization of oligonucleotide-loaded lipid nanoparticles. (Fan Y., et al., 2021)
Fig. 1. Preparation and characterization of oligonucleotide-loaded lipid nanoparticles. (Fan Y., et al., 2021)
Our Encapsulation Efficiency (LNP) Determination Services
Creative Proteomics extends encapsulation efficiency (LNP) determination services to pharmaceutical companies, research institutions, and drug developers. Our services harness advanced technologies and analytical methods to assess the encapsulation efficiency of DNA & RNA drugs within LNPs. Here are some of the key services we offer.
Formulation Design and Optimization
Creative Proteomics assist in the design and optimization of LNP formulations tailored to the specific characteristics of the DNA & RNA drug and the target application. This involves selecting suitable lipids, incorporating targeting ligands (if necessary), and determining the appropriate drug-to-lipid ratio.
Encapsulation Efficiency (LNP) Determination
Creative Proteomics employs various analytical techniques, such as UV-Vis spectroscopy, high-performance liquid chromatography (HPLC), and dynamic light scattering (DLS), to precisely quantify the amount of DNA & RNA drug encapsulated within LNPs. These methods enable accurate determination of encapsulation efficiency and particle size distribution, critical factors affecting drug delivery.
Stability and Release Studies
To ensure the viability of formulated LNP-drug complexes, we also offer stability and release studies. Our services help in predicting the behavior of encapsulated drugs under various physiological conditions and over an extended period.
Quality Control and Validation
Our encapsulation efficiency (LNP) determination services also include comprehensive quality control and validation measures to meet regulatory standards and ensure the reliability of the data generated.
Process of Encapsulation Efficiency (LNP) Determination
The process of encapsulation efficiency (LNP) determination involves several key steps.
LNP Formulation Preparation
First, LNPs are formulated by combining specific lipids with the DNA & RNA drug.
Drug Loading
The prepared LNPs are then subjected to drug loading, where the DNA & RNA drug is introduced into the LNP formulation. The loading process needs to be carefully controlled to achieve optimal encapsulation efficiency.
Separation of Unencapsulated Drug
After drug loading, any unencapsulated drug is separated from the LNPs using techniques such as ultracentrifugation or size exclusion chromatography.
Quantification
The amount of encapsulated drug is quantified using appropriate analytical methods, like UV-Vis spectroscopy or HPLC.
Calculation of Encapsulation Efficiency
Encapsulation efficiency (EE) is calculated using the formula, EE (%) = (Amount of encapsulated drug / Total drug added) x 100.
Data Analysis
The obtained results are analyzed, and the formulation can be optimized based on the encapsulation efficiency and particle size distribution data.
Encapsulation Efficiency (LNP) Determination Application Fields
The application of encapsulation efficiency (LNP) determination is wide-ranging and holds great promise in the field of molecular medicine.
- Improved Pharmacokinetics.
- Personalized Medicine.
- Stability during Storage and Transportation.
- Cancer Treatment.
Creative Proteomics takes pride in being your trusted partner in DNA & RNA drugs encapsulation efficiency (LNP) determination services. Our expertise, state-of-the-art facilities, and commitment to excellence make us the ideal choice for advancing your DNA & RNA drug development projects. If you are interested in our services, please contact us for more detailed information.
Reference
- Fan Y.; et al. (2021). Automated high-throughput preparation and characterization of oligonucleotide-loaded lipid nanoparticles. Int J Pharm. 599:120392.
For research use only, not intended for any clinical use.

 Fig. 1. Preparation and characterization of oligonucleotide-loaded lipid nanoparticles. (Fan Y., et al., 2021)
Fig. 1. Preparation and characterization of oligonucleotide-loaded lipid nanoparticles. (Fan Y., et al., 2021)