Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) is a crucial mechanism of action for therapeutic antibodies. It involves the destruction of target cells through the recruitment of immune cells, such as natural killer (NK) cells, which recognize and eliminate cells coated with antibodies. The measurement of ADCC activity is of significant importance in the development and characterization of antibody-based biotherapeutics. At Creative Proteomics, we offer a comprehensive antibody drug ADCC activity testing service to aid in the evaluation of therapeutic antibody efficacy.
Why is ADCC Activity Testing Necessary?
ADCC is not only the main way for the body to clear cells infected by viruses or other pathogens through antibodies, but also one of the mechanisms of action by which antibody drugs currently exert their clinical effects. In the process of antibody drug development, the critical quality attributes (CQA) that must be characterized by therapeutic antibody drugs include the strength of the candidate antibody drug binding to the target and the degree of binding to the patient's immune system to induce ADCC action. Based on the principle of ADCC mechanism of action, detecting whether an antibody possesses biological activity for ADCC action and further analyzing the strength of the antibody's biological activity for ADCC action has become a crucial step in the development of antibody drugs and their quality control process. Meanwhile, ADCC activity is also one of the ex vivo detection indicators in the clinical trials of biologics and immunomodulatory drugs.
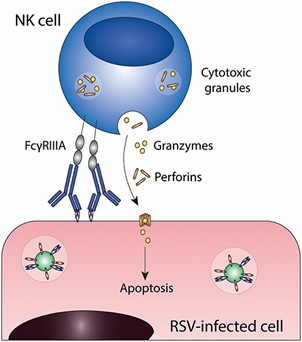 Fig. 1 Antibody-dependent cell-mediated cytotoxicity (ADCC). (Van, Erp. E. A. et al., 2019)
Fig. 1 Antibody-dependent cell-mediated cytotoxicity (ADCC). (Van, Erp. E. A. et al., 2019)
Our ADCC Testing Services
At Creative Proteomics, we provide reliable and highly accurate ADCC activity assays to assess the potency of your therapeutic antibodies. Our experienced team of scientists utilizes state-of-the-art techniques and methodologies to deliver precise and reproducible results.
- Assay design and customization
We understand that each therapeutic antibody requires a tailored approach for ADCC analysis. Our experts work closely with you to design and optimize an ADCC assay that fits your specific requirements. We offer a range of assays, including PBMC assays, natural killer cell assays and reporter gene-based assays, allowing for greater flexibility in experimental design.
- Engineered effector cells
To ensure the assay's reliability and sensitivity, we employ highly characterized, engineered effector cells. These cells possess enhanced ADCC activity, allowing for the detection of subtle differences between samples.
- Precise target cell line selection
Choosing the appropriate target cell line is crucial to mimic the in vivo conditions accurately. Our team will help you select the most suitable target cells based on their expression levels of target antigens, compatibility with effector cells, and other relevant factors, ensuring optimal assay performance.
- High-quality assay readouts
We employ advanced flow cytometry techniques and multi-parametric analysis which ensures high-quality data and reliable results, enabling you to make informed decisions during drug development.
ADCC Testing Service Features
- We utilize a wide variety of target cell lines expressing the specific antigen of interest to ensure accurate and relevant results.
- Our testing protocols follow standardized industry practices and guidelines to maintain reliability and reproducibility across multiple experiments.
- Our testing services are conducted with a quick turnaround time, allowing you to access results promptly.
Creative Proteomics is committed to providing customized options to meet your specific needs, including different target cell types, effector cell sources, or modifications to the assay protocol. Our flexible services allow you to customize your ADCC activity assay to your unique requirements. Contact us to learn more about how our antibody drug ADCC activity testing service can contribute to the success of your drug development efforts.
Reference
- Van, Erp. E. A.; et al. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Frontiers in immunology. 2019, 10: 548.

 Fig. 1 Antibody-dependent cell-mediated cytotoxicity (ADCC). (Van, Erp. E. A. et al., 2019)
Fig. 1 Antibody-dependent cell-mediated cytotoxicity (ADCC). (Van, Erp. E. A. et al., 2019)