Antibody Fusion Protein Drug
Online Inquiry
Antibody fusion proteins, as popular therapeutic drugs, combine the excellent specificity and pharmacokinetic properties of antibody drugs, enabling targeted delivery of payloads and achieving excellent therapeutic effects. At Creative Proteomics, we specialize in providing customized characterization services for antibody fusion protein drugs to ensure their quality, safety and efficacy. Our advanced technology and expertise guarantee that we deliver precise and comprehensive analytical results that offer valuable insights for the development and introduction of antibody fusion protein-based drugs to our clients.
Introduction of Antibody Fusion Protein Drugs
Antibody fusion proteins are recombinant proteins that combine antibody fragments with biologically active functional proteins. CD4-Fc fusion proteins were the first such proteins to emerge. CD4-Fc fusion proteins were the initial fusion proteins to emerge. It prevents HIV-1 from infecting T cells and monocytes. This technique of using genetic recombination to link a drug protein gene with a specific carrier protein gene through a linker gene and then co-expressing it to obtain the target protein after transferring it to a biological expression system is known as fusion protein expression technology. Depending on the drug carrier protein, fusion proteins are generally categorized into three types:
- Fusion with long half-life proteins such as lgG Fc, HAS and transferrin.
- Fusion with negatively charged peptides, e.g. CTP, etc.
- Fusion with non-structural peptides, such as ELP, etc.
Currently, Fc fusion proteins are the mainstay of the market, which have a broad therapeutic prospect.
Antibody Fusion Protein Drug Characterization Services Provided by Creative Proteomics
Due to the complexity and heterogeneity of the structure of Fc fusion proteins, many different analytical tools are required for analytical characterization and quality control. Examples include charge variants, volume variants, glycan profiles and key physicochemical properties such as identification, purity and integrity. The main analytical methods involved are ion exchange chromatography, capillary isoelectric focusing, hydrophobic and reversed-phase chromatography, mass spectrometry-based peptide mapping, size exclusion chromatography, capillary gel electrophoresis, hydrophilic chromatography and other analytical methods. Creative Proteomics can provide customized characterization services for different antibody fusion protein drugs to ensure comprehensive analysis of multiple quality attributes of the drug.
Our characterization services are carried out in two main areas: antibody protein and fusion protein stock release assays, and the main assays and methods include the following:
Antibody protein characterization list
| Quality Attributes |
Testing Items |
Method |
| Primary structure |
Molecular weight determination |
LC-MS |
| Amino acid sequence |
| Post-translational modifications |
| C/N-terminal sequence analysis |
| Free sulfhydryl group |
Zymometric assay |
| N-terminal amino acid sequence |
Edman N |
| High order structure |
Disulfide bonding |
LC/MS |
| Secondary/tertiary structures |
CD |
| Thermal stability |
DSC |
| Charge heterogeneity |
electric charge heterogeneity |
IEX/cIEF |
| Molecular size heterogeneity |
molecular size heterogeneity |
SEC/CZE |
| Glycosylation |
Glycosylation site |
LC/MS |
| Glycoform distribution |
HPLC/LC-MS |
| Sialic acid content |
HPLC-FLD |
| Bioactivity |
Fab binding |
Zymometric assay |
| Fac binding |
Affinity assay |
| ADCC |
Zymometric assay |
| CDC |
Zymometric assay |
Antibody fusion protein release tests list
Why Choose Us?
- A broad portfolio of characterization services ensures a comprehensive understanding of the properties of antibody fusion protein drugs, including quality, efficacy, and safety.
- Customized characterization strategies and analytical methods are available to meet client-specific project requirements.
- Advanced platform technology combined with our extensive project experience ensures detailed and reliable characterization data.
- Strict adherence to regulatory guidelines and quality standards ensures that the data provided can effectively support the development and approval of antibody fusion protein drugs.
- Expert advice, data interpretation and decision-making guidance throughout the project.
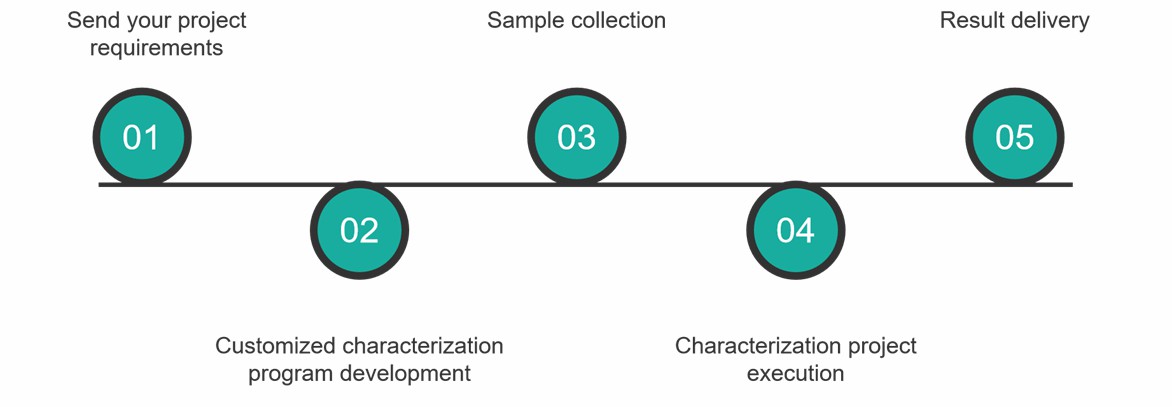
Characterization Service Process
At Creative Proteomics, you can get comprehensive antibody fusion protein drug characterization services by following these simple steps.
Creative Proteomics is committed to providing you with high-quality antibody fusion protein drug characterization services to support your drug development and manufacturing projects. Contact us to learn more about how our characterization services can help you achieve your antibody fusion protein drug development goals.


