Oxidation is the most significant post-modification problem encountered with monoclonal antibody (mAb) drugs. Oxidation of antibody drugs represents a major degradation pathway, which may impact bioactivity, serum half-life, and colloidal stability. At Creative Proteomics, by leveraging cutting-edge technology and a team of highly skilled scientists, we strive to provide accurate and timely analysis of oxidized post-translation modification (PTMs) to support the development and characterization of antibody drugs.
Introduction of Amino Acid Oxidation
Oxidation could occur through the presence of heavy metal ions, reactive impurities in excipients, light, or simply the presence of oxygen. The generation of peroxides such as polysorbate also introduce the risk for oxidation through autoxidation. It can occur at almost all stages of mAb development, e.g. during cell culture, purification, storage, and even during analytical assays. Oxidation of methionine and tryptophan have been found to be a major instability factor of pharmaceutical proteins. Oxidation of antibody therapeutics could cause changes in the hydrophobic nature and may introduce structural alterations. Oxidation of the methionine positions 252 and 428 weaken the FcRn6, 16 and Fcγ17 receptor binding, which could consequently shorten serum half-life. Furthermore, the bioactivity, target binding,6 and stability may also be compromised, and impact on aggregation has been documented.
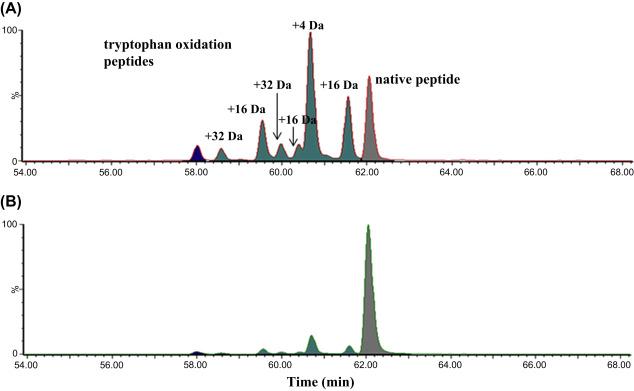 Fig. 1 Representative extracted ion chromatogram of the oxidized forms of a tryptophan-containing mAb peptide following forced oxidation with 1.2 mM AAPH. (Robotham, A. C., et al, 2020)
Fig. 1 Representative extracted ion chromatogram of the oxidized forms of a tryptophan-containing mAb peptide following forced oxidation with 1.2 mM AAPH. (Robotham, A. C., et al, 2020)
Oxidation Analysis Services at Creative Proteomics
Oxidation occurs on a variety of amino acid residues, the most susceptible to oxidative modification being methionine (Met), which is oxidized to form methionine sulfoxide. Using liquid chromatography-mass spectrometry (LC-MS) based methods, Creative Proteomics offers the following analytical services to our clients:
- Identify methionine oxidation sites.
- Assess the degree of oxidation of each methionine.
- Characterization of site-specific oxidation products.
- Evaluate the effect of methionine oxidation on antibody structural stability and potential immunogenicity.
What We Deliver?
| Related mass spectrometer parameters |
Mass spectra |
| Raw data |
Nature and extent of oxidative modifications present in samples |
One-Stop Analysis Process at Creative Proteomics
Creative Proteomics is committed to providing one-stop oxidation analysis services using our high-throughput and high-sensitivity analytical technologies. All you need to do is to submit your samples to us and our scientists will work closely with you to deliver the final results in a short period of time. Our one-stop analytical process consists of the following steps:
- Sample preparation.
- Oxidation induction: We subject the antibody drug samples to controlled oxidative stress, mimicking the conditions they may encounter during storage or handling.
- Separation and Purification: We perform high-performance liquid chromatography (HPLC) to separate the oxidized antibody drug from the unoxidized ones.
- Mass spectrometry analysis: Identifying and characterizing the specific oxidative modifications, such as oxidation of amino acids (methionine, cysteine, tryptophan), glycation, deamidation, and disulfide bond formation.
- Analysis of the effects of oxidation on the structure, stability, aggregation and function of therapeutic antibodies.
- Data analysis and interpretation.

Creative Proteomics provides our clients with authentic, reliable and timely characterization analysis with our advanced technology platform. Contact us to learn more about our oxidation analysis services. We will be happy to assist you.
Reference
- Robotham, A. C.; et al. LC-MS characterization of antibody-based therapeutics: recent highlights and future prospects. Approaches to the Purification, Analysis and Characterization of Antibody-Based Therapeutics. Elsevier. 2020: 1-33.

 Fig. 1 Representative extracted ion chromatogram of the oxidized forms of a tryptophan-containing mAb peptide following forced oxidation with 1.2 mM AAPH. (Robotham, A. C., et al, 2020)
Fig. 1 Representative extracted ion chromatogram of the oxidized forms of a tryptophan-containing mAb peptide following forced oxidation with 1.2 mM AAPH. (Robotham, A. C., et al, 2020)