A monoclonal antibody (mAb) is defined as a glycoprotein capable of binding an antigen to a specific epitope. In recent years, it has become increasingly therapeutically sophisticated, with a variety of monoclonal antibody drugs receiving regulatory approval. However, the activity, efficacy and immunogenicity of monoclonal antibody are influenced by their structure and quality. How to fully characterize the key quality attributes of antibody drugs has become a critical point and difficulty in antibody drug development. The development and production process requires the development of robust methods for the comprehensive characterization of key quality attributes such as structural and physicochemical properties, biological activity, purity, and stability of monoclonal antibody drugs.
Creative Proteomics provides one-stop analytical characterization services for monoclonal antibody drug research and development across a range of technologies, including biochemistry, physical chemistry and bioassays. Our dedicated assay team and advanced analytical equipment ensure that we can provide our clients with complete, reliable analytical solutions to accelerate their monoclonal antibody drug screening and development programs.
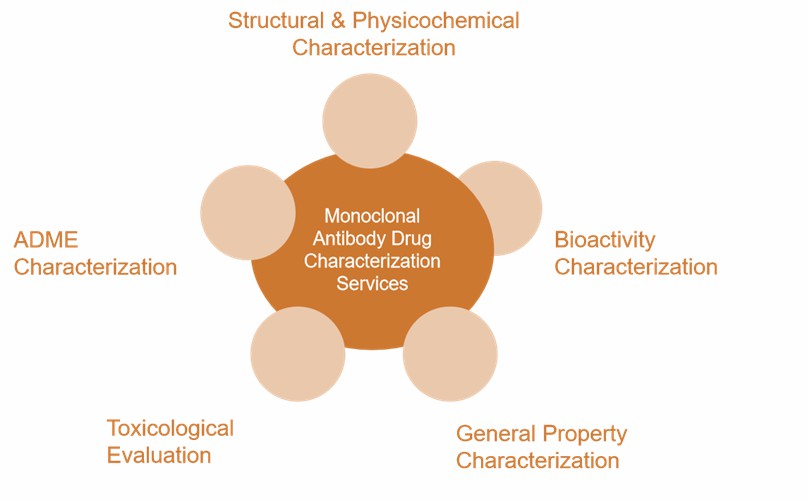
Monoclonal Antibody Drug Characterization Services Scope
At Creative Proteomics, we provide comprehensive analytical and characterization services for monoclonal antibody drug from multiple dimensions to support drug development programs and ensure regulatory compliance. We provide accurate and detailed drug characterization covering physicochemical properties, biological activity, immunological properties, pharmacokinetics, purity, impurities, stability, and safety studies in the following areas:
Comprehensive analysis of the primary structure, post-translational modifications, advanced structure, charge heterogeneity, aggregates and other physicochemical properties of monoclonal antibodies.
The content and drug potency of the active ingredients of monoclonal antibody drugs were determined using commonly used biological activity assays including ADCC, CDC, inhibition of cell proliferation, cytotoxicity, cytokine secretion capacity, internalization, etc.
Comprehensive characterization of basic attributes such as appearance, purity, safety and stability of monoclonal antibody.
Assessment of possible immunotoxicity and safety risks of monoclonal antibody drugs by in vivo and in vitro experiments.
We design experiments to study the metabolism and distribution of monoclonal antibody drugs according to their target types, pharmacokinetic characteristics and other factors, and provide ADME data for monoclonal antibody drugs.
Monoclonal Antibody Drug Characterization Services Provided by Creative Proteomics
For the analytical characterization of monoclonal antibody drug, our team of experts provides customized solutions. Advanced analytical equipment is utilized and strict protocols are followed to obtain accurate and reliable results.
- Intact molecular weight (MW) and MW distribution (mass spectrometry, SEC-MALS)
- Amino acid sequence and composition (peptide mapping, mass spectrometry, amino acid analysis)
- Glycan profiling (HILIC-FLR-MS)
- Extinction coefficient determination and validation
- Electrophoretic and isoform patterns (cIEF, gel IEF, SDS PAGE, Western Blot, CZE)
- Liquid chromatographic patterns (RP-HPLC, Ion Exchange HPLC, SEC)
- Spectroscopic profile (CD, NMR, FTIR, Fluorescence) for secondary and higher order structure
- Disulphide bridge mapping (Mass spectrometry)
- Terminal amino acid sequencing including lysine clipping, proline amidation, pyroglutamic acid. (Mass spectrometry, CE)
- Evaluation of PTMs including oxidation and deamidation (Mass spectrometry, cIEF)
- Potency assessment (cell-based assays)
- Aggregation studies (SEC, DLS, Western Blot)
- Forced degradation studies
- Quantification of host cell proteins (HCP) - total or individual HCPs and residual DNA
- Physicochemical properties (includes colour, clarity / opalescence, pH, particulates, turbidity, extractable volume, moisture, osmolality) comms
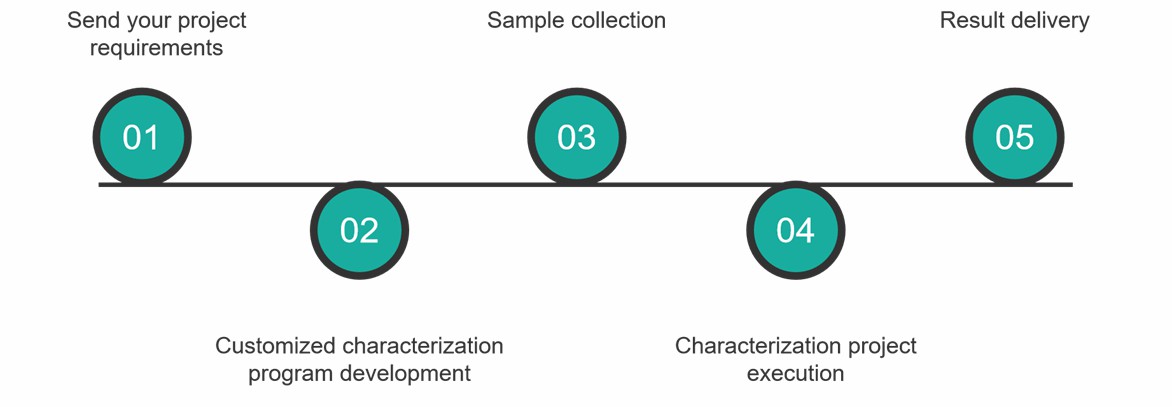
Characterization Service Process
At Creative Proteomics, you can get a comprehensive characterization service by following these simple steps.
Effective early evaluation of antibody drug allows for more comprehensive consideration of issues that may affect the expected end result of a stable drug product, thus saving time and costs. Creative Proteomics is your trusted partner in providing fast turnaround times and personalized characterization services for your monoclonal antibody drug development projects to meet the demands of our clients. Contact us to learn more about our monoclonal antibody drug analysis and characterization services, and how we can assist you with your drug development demands.



