scfv Like Antibody Fragment
Online Inquiry
Single-chain fragment variable (scFv) is the smallest functional structural unit that possesses all the antigen-binding specificity of the parental antibody, consisting of an elastic linking peptide (linker) that connects the heavy chain to the light chain of the antibody's variable region. scFv has a wide range of applications in antiviral, tumor therapy, autoimmune disease therapy, targeted drug therapy, and so on. Creative Proteomics provides customized characterization services for ScFv fragment antibody drugs to support the development and optimization of ScFv fragment antibodies as antibody drugs.
Introduction of scFv
scFv is a small molecule genetically engineered antibody consisting of the heavy chain variable region and the light chain variable region of the antibody connected by a short peptide of 15-20 amino acids and does not contain an Fc fragment. The complete antibody consists of two heavy chains (H) and two light chains (L), which can be artificially modified to express only the variable region. Antibodies can be artificially modified to express only the variable region. Antibodies expressed from recombinant genes that link the variable region of the heavy chain (VH) to the variable region of the light chain (Vu) are called single-chain antibodies (scFv) by means of a synthetic linker peptide gene. The scFv antibody fragments can function as antibody drugs alone, or they can be linked to each other or even subjected to further modifications such as PEG modification or radiolabeling. The currently approved scFv is brolucizumab, which targets VEGF-A, for the treatment of wet age-related macular degeneration.
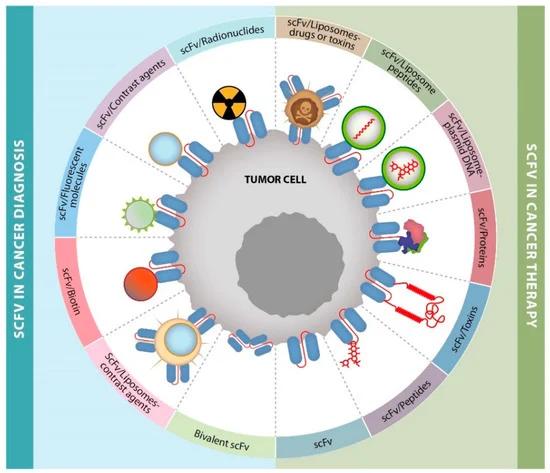 Fig. 1 ScFv applications in cancer diagnosis or therapy. (Muñoz-López, P. et al., 2022)
Fig. 1 ScFv applications in cancer diagnosis or therapy. (Muñoz-López, P. et al., 2022)
scFv-Like Antibody Fragment Characterization Services at Creative Proteomics
Creative Proteomics is deeply involved in the field of antibody drug characterization and has established a comprehensive analytical technology platform with a full suite of antibody drug characterization solutions. Our characterization services cover a wide range of analyses to ensure that we help our clients thoroughly understand the key quality attributes of their ScFv fragment antibody-based drugs. Below is an overview of our services:
- Structural characterization: we help our clients evaluate the primary and higher-order structures of ScFv-based antibody drugs through high-resolution structural analysis techniques that provide insights into the quality, stability and efficacy of the drugs.
- Physicochemical characterization: using a combination of analytical techniques, we can perform a comprehensive analysis of the physicochemical properties of ScFv-based antibody drugs, including post-translational modifications, glycosylation patterns, charge variants, and more. These results can provide structurally relevant results for further evaluation of drug quality, efficacy and stability.
- Binding activity and affinity assessment: we used bioanalytical techniques based on surface plasmon resonance (SPR) as well as enzyme-linked immunosorbent assay (ELISA) to perform comprehensive analyses of the binding and functional activities of ScFv-based antibody drugs. These analyses provide comprehensive insights into the biological activity of ScFv fragment antibody-based drugs.
- Stability assessment: the stability of ScFv-based antibody drugs was assessed by subjecting them to various stress conditions and analyzing key quality attributes such as thermal stability, aggregation propensity, and conformational integrity to evaluate the stability of this class of drugs.
- Immunogenicity assessment: we conduct comprehensive assessments of the immunogenicity of ScFv-based antibody drugs by analyzing the results of in vivo and in vitro experiments together.
- Batch-to-batch consistency assessment: by rigorously testing the drug for purity, impurities, product-related variants and critical quality attributes, we can provide information on lot-to-lot consistency of the product.
Characterization Service Process
At Creative Proteomics, you can get comprehensive small molecule antibody drug characterization services by following these simple steps.
Creative Proteomics' antibody drug characterization platform is equipped with a team of experienced experts and advanced characterization technologies. We are committed to providing customized characterization services to accelerate our clients' drug development process based on their unique demands. Contact us for more information and we will be happy to assist you.
Reference
- Muñoz-López, P.; et al. Single-chain fragment variable: recent Progress in cancer diagnosis and therapy. Cancers. 2022, 14(17): 4206.

 Fig. 1 ScFv applications in cancer diagnosis or therapy. (Muñoz-López, P. et al., 2022)
Fig. 1 ScFv applications in cancer diagnosis or therapy. (Muñoz-López, P. et al., 2022)