DAR Distribution Characterization
Online Inquiry
Among a variety of properties of an ADC, the drug-to-antibody ratio (DAR) and drug load distribution should be well characterized for their impact on drug efficacy and safety. Creative Proteomics employs advanced analytical techniques and establishes appropriate methods to help clients characterize the DAR distribution of different ADCs. The accurate results obtained will provide valuable insights for ADC pharmacokinetic and mechanism of action studies, thus facilitating the successful development of ADC drugs.
Significance of DAR Distribution Characterization
DAR distribution is defined as the stoichiometric distribution of the number of conjugated drugs present on the antibody carrier. Different drug distribution may contribute to very different pharmacokinetics and toxicological properties and can directly affect the efficacy and safety of ADCs. Furthermore, the ADCs distributed (either in cells or in body) after administration, and how they accumulated in the target tissues are also very crucial for understanding the working mechanisms of ADCs. It can indicate how ADCs interact with cells, how they are up taken by cells, and finally how they are removed from the body. Such information cannot be obtained by conventional in vitro analytical tools. Therefore, the DAR distribution must be adequately characterized using advanced techniques combined with in vivo modeling to ensure the efficacy and safety of ADCs.
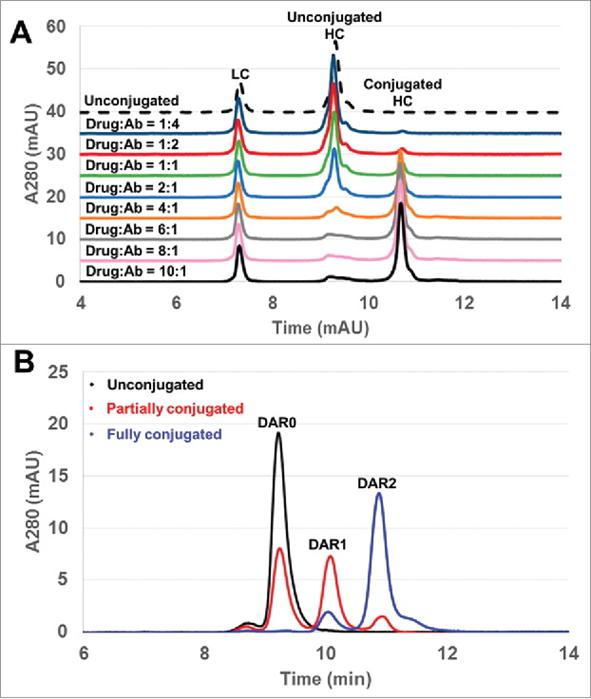 Fig. 1 The reduced RP-HPLC method was used as a tool to monitor DAR distribution. (Wagh, A., et al., 2014)
Fig. 1 The reduced RP-HPLC method was used as a tool to monitor DAR distribution. (Wagh, A., et al., 2014)
DAR Distribution Characterization Methods at Creative Proteomics
Creative Proteomics' DAR distribution characterization services include a variety of advanced analytical techniques to efficiently assess the distribution patterns and quantification of drug payloads within target tissues and organs in preclinical and clinical settings. We provide the most appropriate assays based on our clients' demands.
| Determination Methods |
Description |
Advantages |
| Capillary electrophoresis (CE) |
Capillary electrophoresis utilizes the principle of electrophoretic mobility to separate and quantify the individual components of an ADC based on their mass-to-charge ratio. This method allows precise determination of the distribution of drug molecules attached to the antibody. |
- High sensitivity.
- Fast and efficient, allowing for rapid analysis and high throughput screening of ADCs.
|
| Mass Spectrometry Imaging (MSI) |
MSI enables direct imaging and quantification of drug payloads in tissues and organs, precise quantification of drug concentrations, and the establishment of concentration-time curves. |
- High spatial resolution, enabling the visualization of drug distribution patterns at the cellular level.
|
Features of Our DAR Distribution Characterization Services
- Customized characterization solutions based on customer's sample requirements.
- Advanced DAR distribution analysis techniques and equipment ensure accurate and reliable results.
- A comprehensive analysis report describes the DAR distribution of the sample, including detailed graphical representation and statistical analysis.
Creative Proteomics' team of experienced scientists and technicians are well versed in the latest methods of DAR distribution analysis, ensuring the highest quality of service. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Wagh, A.; et al. Challenges and new frontiers in analytical characterization of antibody-drug conjugates[C]//MAbs. Taylor & Francis. 2018, 10(2): 222-243.

 Fig. 1 The reduced RP-HPLC method was used as a tool to monitor DAR distribution. (Wagh, A., et al., 2014)
Fig. 1 The reduced RP-HPLC method was used as a tool to monitor DAR distribution. (Wagh, A., et al., 2014)