DAR Value Determination
Online Inquiry
Various DAR (drug-to-antibody ratio) species could carry different efficacy and safety, the DAR value is a critical parameter for ADCs. Due to the complexity and heterogeneity of ADCs, traditional protein analysis methods cannot be directly applied to the DAR determination of ADCs. At Creative Proteomics, we combine advanced assay technologies to provide our clients with accurate and comprehensive DAR measurements that provide insights into the safety and efficacy assessment of ADCs.
Impact of DAR on The Safety and Efficacy of ADCs
DAR (drug-to-antibody ratio) is defined as the average of the amount of drug carried by an antibody, and determines the "payload" that can be delivered to a tumor, which directly affects the safety and efficacy of an ADC. However, a higher DAR is not always better. Although a high DAR has a greater drug delivery capacity, it is also more likely to be targeted by the body's immune system and thus be removed from the body as a foreign body, reducing the effectiveness of the ADCs. As a result, most ADC drugs are limited to a DAR between 2-4. How to keep the DAR value at a suitable point, both efficacy and effectiveness, is the key to successful development of ADCs.
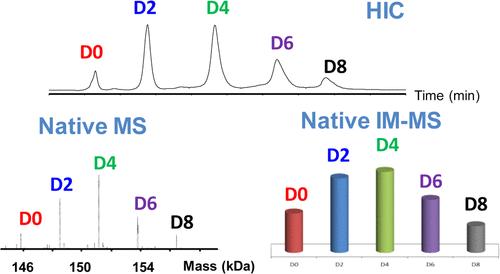 Fig. 1 High resolution native MS and IM-MS for average DAR and DAR distribution assessment. (Debaene, F., et al., 2014)
Fig. 1 High resolution native MS and IM-MS for average DAR and DAR distribution assessment. (Debaene, F., et al., 2014)
DAR Value Determination Methods at Creative Proteomics
Currently, Creative Proteomics has developed several methods for quantifying DAR values in ADCs. Each of these methods has its own advantages, and our experienced experts will select the best determination method for our customers based on different drug properties and the structure of the binding site and linker.
| Determination Methods |
Description |
Advantages |
| Ultraviolet-Visible (UV/Vis) spectroscopy |
The Ultraviolet-Visible (UV/Vis) spectroscopy is a relatively simple method for DAR determination. UV/Vis is based on the technique of protein concentration determination. Using the absorbance of the ADC at the appropriate wavelength and the extinction coefficient of the antibody and payload, the average DAR can be determined. |
- Simple and easy to use without the need for complex sample preparation and establishment of HPLC or MS conditions.
- Suitable for the determination of DAR values for a wide range of ADCs.
|
| Mass spectrometry-based analysis |
Mass spectrometric methods are suitable for the determination of DAR values of lysine-coupled ADCs, including liquid chromatography tandem mass spectrometry and MALDI-TOF-MS. After antibody coupling on different numbers of cytotoxic small molecules, ADCs show molecular heterogeneity, and different DAR substances have different mass-to-charge ratios (m/z) analyzed by high-resolution mass spectrometry, and then the average DAR value is calculated based on the peak intensity or peak area of the corresponding DAR mass spectrometry signals. |
- High resolution.
- High sensitivity.
|
Features of Our DAR Value Determination
- A team of experts who are well versed in DAR measurement methods.
- State-of-the-art equipment and techniques for accurate DAR value determination.
- Customized, flexible testing solutions that meet specific customer requirements.
Creative Proteomics is committed to ensuring that our clients receive the best possible assay results with a flexible, adaptable approach. Our experts will assist clients with data analysis and provide insights for optimizing the design and dosing of ADCs. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Debaene, F.; et al. Innovative native MS methodologies for antibody drug conjugate characterization: high resolution native MS and IM-MS for average DAR and DAR distribution assessment. Analytical chemistry. 2014, 86(21): 10674-10683.

 Fig. 1 High resolution native MS and IM-MS for average DAR and DAR distribution assessment. (Debaene, F., et al., 2014)
Fig. 1 High resolution native MS and IM-MS for average DAR and DAR distribution assessment. (Debaene, F., et al., 2014)