Title: Quantitative subcellular acyl-CoA analysis reveals distinct nuclear metabolism and isoleucine-dependent histone propionylation
Journal: Molecular Cell
Published: November 2021
Background
The study addresses the challenges of quantitatively measuring metabolites in subcellular compartments to understand their roles in cellular processes. Metabolism is highly compartmentalized, and current whole-cell analysis methods provide limited compartment-specific insights. Acyl-coenzyme A (acyl-CoA) thioesters, involved in various metabolic pathways, were the focus of this study. Specifically, the research developed a new method called Stable Isotope Labeling of Essential Nutrients in Cell Culture-Subcellular Fractionation (SILEC-SF), which allows for the quantification of acyl-CoAs in subcellular compartments. This method uses isotope-labeled internal standard controls to correct for analytical challenges and disruptions during fractionation. The study found that the nuclear acyl-CoA profile is distinct from that of the cytosol, with nuclear enrichment of propionyl-CoA. Using isotope tracing, isoleucine was identified as a key metabolic source of nuclear propionyl-CoA and histone propionylation, revealing a novel mechanism connecting metabolism and the epigenome.
Materials & Methods
The study developed and used a method called Stable Isotope Labeling of Essential Nutrients in Cell Culture-Subcellular Fractionation (SILEC-SF) for accurate quantification of metabolites in subcellular compartments. The method utilizes isotope-labeled internal standards that are incorporated into CoA, which can be detected by liquid chromatography-mass spectrometry (LC-MS).
SILEC-SF Process:
SILEC labeling with 15N1 13C3-Vitamin B5 (VB5) results in the incorporation of the isotope label into CoA. SILEC cells are harvested in fractionation buffer and mixed with experimental samples prior to fractionation, ensuring the presence of the internal standard before and after fractionation. Each subcellular compartment is separated, and the metabolites are extracted and analyzed by LC-MS. The ratio of signal intensities from the experimental sample (light) and the isotope-labeled internal standard (heavy) determines the relative quantities of metabolites.
Calibration and Standard Curves:
Separate standard curves were generated for each subcellular fraction by fractionating SILEC internal standard cells in parallel with experimental samples. Known quantities of unlabeled standards were added to generate the curves. These standard curves varied across fractions due to factors such as enrichment of specific acyl-CoA species, extraction efficiency, and matrix effects. The impact of different subcellular matrices on signal intensity was assessed, showing matrix-specific effects that influenced standard curve variation, highlighting the importance of matrix-matched calibration.
SILEC-SF can be adapted for use with different fractionation techniques, including differential centrifugation and immunoprecipitation of organelles. This approach ensures rigorous metabolite quantification in subcellular compartments, making it effective for studying compartmentalized metabolism.
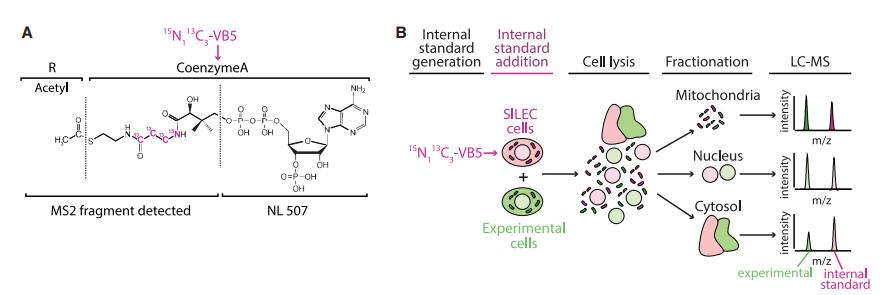 SILEC-SF uses whole-cell internal standards to quantify acyl-coenzyme As (acyl-CoAs) in subcellular compartments
SILEC-SF uses whole-cell internal standards to quantify acyl-coenzyme As (acyl-CoAs) in subcellular compartments
Results
Distinct Acyl-CoA Profiles in Mitochondria and Cytosol:
- SILEC-SF was used to profile acyl-CoA distribution across various cell types and tissues, including adipocytes, fibroblasts, mouse liver, and human heart.
- Acyl-CoA species such as succinyl-CoA, acetyl-CoA, and CoASH were found to be abundant in the cytosol, with succinyl-CoA predominantly present in mitochondria.
- The method was further validated by mitochondrial immunoprecipitation (Mito-IP), confirming compartment-specific acyl-CoA distribution.
Mitochondrial Response to Hypoxia:
- Under hypoxia, mitochondrial succinyl-CoA was significantly reduced, while acetyl-CoA remained relatively unaffected in mitochondria but decreased in the cytosol.
- SILEC-SF was able to detect compartment-specific changes in acyl-CoA levels, demonstrating its sensitivity to metabolic perturbations.
Improvement in Quantification Over Conventional Methods:
- SILEC-SF showed superior reproducibility and accuracy compared to traditional internal standard addition methods post-fractionation.
- It accounted for post-harvest metabolism, ensuring precise quantification of acyl-CoA levels in different subcellular compartments.
Cytosolic HMG-CoA Sensitivity to Acetate Supply:
- SILEC-SF revealed that cytosolic HMG-CoA is highly sensitive to acetate availability, particularly in cells deficient in ACLY, highlighting the metabolic regulation of acyl-CoA pools by external substrates.
Nuclear Acyl-CoA Profiles and Propionyl-CoA Enrichment:
- SILEC-SF identified distinct nuclear acyl-CoA profiles, with propionyl-CoA being highly enriched in the nucleus.
- This finding suggested a unique metabolic environment in the nucleus, which might influence chromatin modification processes.
Isoleucine Catabolism Contributes to Nuclear Propionyl-CoA:
- Isoleucine was identified as a key contributor to nuclear propionyl-CoA generation, as isotope tracing showed significant incorporation of isoleucine-derived propionyl-CoA in the nucleus.
- This propionyl-CoA was also found to be a substrate for histone propionylation (Kpr) marks.
Impact of BCAA Deprivation on Nuclear Propionyl-CoA and Kpr Marks:
- Deprivation of branched-chain amino acids (BCAAs), particularly isoleucine and valine, resulted in a significant reduction in nuclear propionyl-CoA levels.
- A corresponding decrease in histone Kpr marks at specific sites was observed, confirming that nuclear propionyl-CoA availability regulates Kpr modifications.
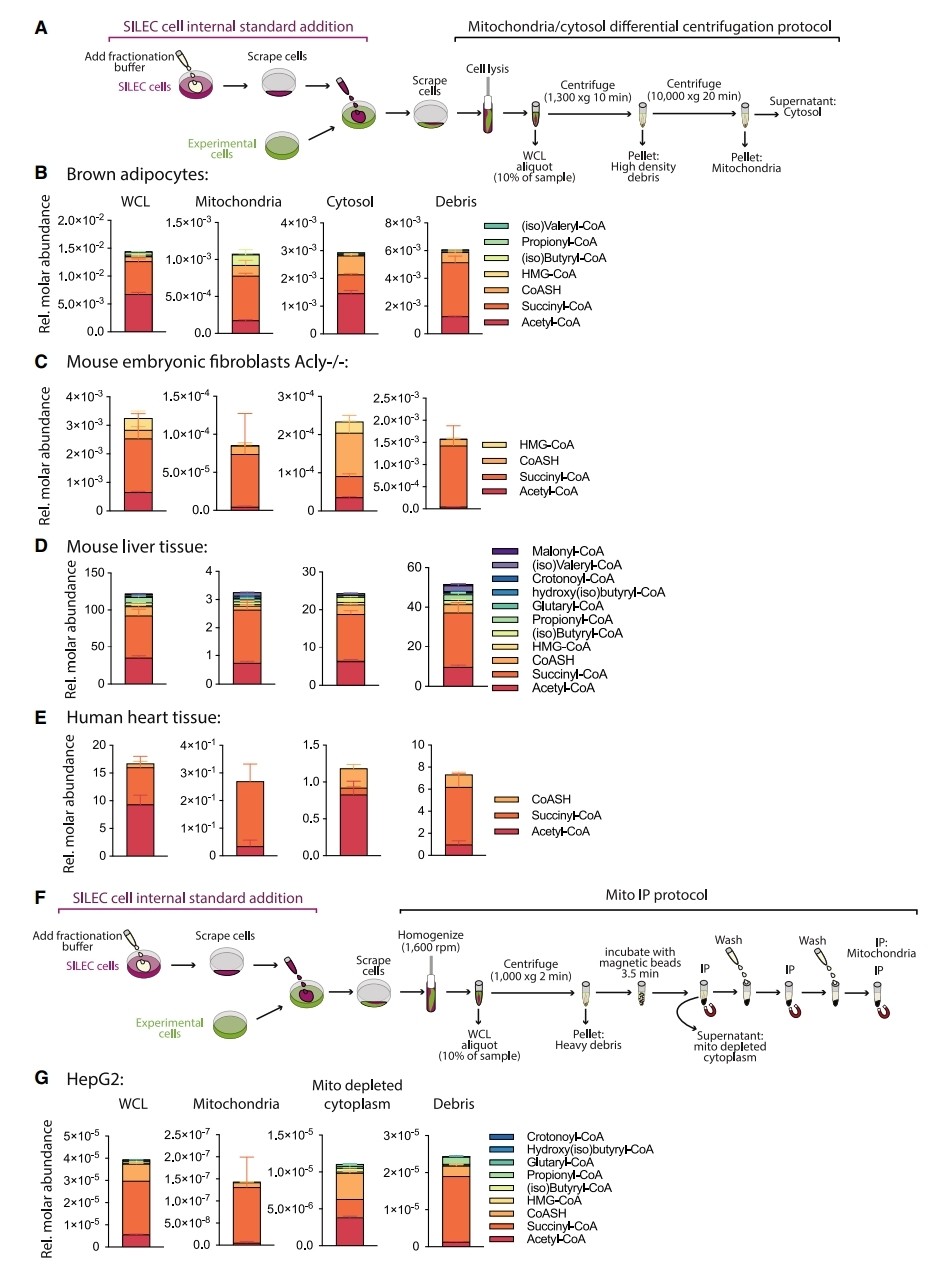 SILEC-SF reveals compartment-specific acyl-CoA profiles
SILEC-SF reveals compartment-specific acyl-CoA profiles
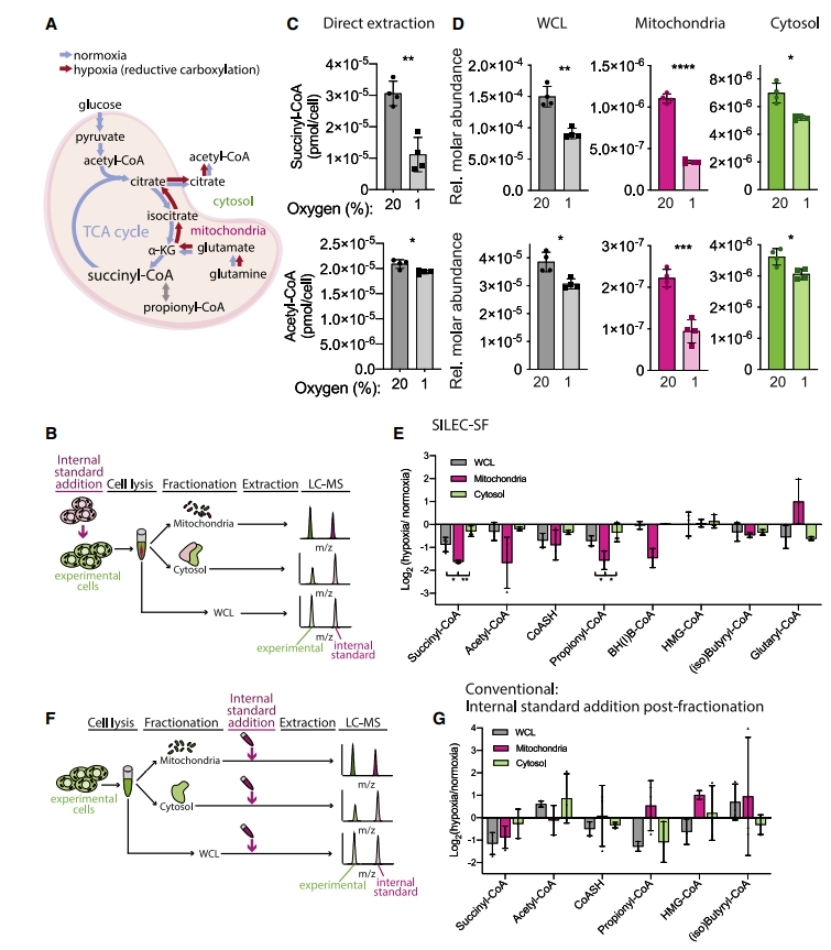 SILEC-SF detects distinct mitochondrial response to hypoxia
SILEC-SF detects distinct mitochondrial response to hypoxia
Reference
- Trefely, Sophie, et al. "Quantitative subcellular acyl-CoA analysis reveals distinct nuclear metabolism and isoleucine-dependent histone propionylation." Molecular cell 82.2 (2022): 447-462. https://doi.org/10.1016/j.molcel.2021.11.006




