Title: Sensitive determination of malondialdehyde in rat prostate by high performance liquid chromatography with fluorescence detection
Journal: Scientific Reports
Published: 2020
Background
Malondialdehyde (MDA) is a reactive product formed during oxidative stress, resulting from the peroxidation of polyunsaturated fatty acids by reactive oxygen species (ROS). It plays a crucial role in the pathogenesis of various diseases, including cancer, diabetes, cardiovascular, and liver diseases, and is used as an indirect marker of oxidative damage. However, accurately determining MDA levels is challenging due to its low concentration and interference from other compounds in biological samples. Traditional methods such as spectrometry and gas chromatography-mass spectrometry (GC-MS) face limitations in sensitivity and specificity. To improve detection, derivatization techniques are often used, but existing methods still struggle with low sensitivity or lengthy procedures. This study addresses these challenges by developing a sensitive and selective high-performance liquid chromatography (HPLC) method for determining MDA using a novel fluorescent derivatization reagent, N-acetylhydrazine acridone (AHAD), offering improved sensitivity, selectivity, and simplicity.
Materials & Methods
Chemicals and Reagents:
- 1,1,3,3-Tetraethoxypropane (Shanghai Aladdin Bio-Chem Technology, 97% purity)
- N-acetylhydrazine acridone (AHAD, synthesized in-house)
- Acetonitrile, methanol (HPLC grade, Anaqua Chemical Supply)
- Phosphoric acid, hydrochloric acid, sodium hydroxide, dimethylformamide, potassium carbonate, acetic acid, sulfuric acid, trichloroacetic acid (analytical grade, Sinopharm Chemical Reagent)
- Acridone (Maya-reagent)
- Pure water (Milli-Q system)
Equipment:
- Agilent 1260 series HPLC system (G1311B quaternary pump, G1329B autosampler, G1316A thermostated column compartment, G1321B fluorescence detector)
- Agilent ZORBAX SB-C18 column (250 mm × 4.6 mm, 5 μm)
Chromatographic Conditions:
- Eluents: A – water, B – acetonitrile
- Gradient elution: 0–10 min, 20% B to 45% B; 10–15 min, 45% B to 100% B
- Flow rate: 1.0 mL/min
- Fluorescence detection: Excitation 370 nm, Emission 420 nm
- Column temperature: 30 °C
Synthesis of AHAD:
1. 2-(9-Acridone)-acetic acid (10 mmol) was refluxed with methanol (100 mL) and concentrated sulfuric acid (5 mL) for 4 hours, filtered, and dried to yield 2.38 g (89.1% yield).
2. 2-(9-Acridone)-ethyl acetate (5 mmol) was refluxed with ethanol (30 mL) and hydrazine hydrate (10 mL) for 6 hours, cooled, filtered, and recrystallized to yield 0.91 g (68.2% yield).
Solution Preparation:
- AHAD stock solution: 26.8 mg in 10 mL acetonitrile (0.01 mol/L).
- MDA standard stock solution: 292 μL of 1,1,3,3-tetraethoxypropane in 100 mL 1% sulfuric acid (0.012 mol/L).
- MDA solutions for derivatization and HPLC prepared by dilution with water. Stored at 4°C.
Samples:
- Six male rats were decapitated, and the prostate gland was carefully dissected, homogenized, and centrifuged to obtain the prostate gland extract. Stored at −80°C until analysis.
Derivatization Procedure:
- For standard MDA: 250 μL AHAD solution, 100 μL MDA solution, and 100 μL trichloroacetic acid (1.0 mol/L) were mixed in an ampoule, sealed, and incubated at 80°C for 30 minutes. After cooling, the mixture was diluted to 1.0 mL with water.
- For prostate extract: 100 μL extract, 100 μL trichloroacetic acid, and 250 μL AHAD were mixed, incubated at 80°C for 30 minutes, cooled, diluted to 1.0 mL, and centrifuged. The supernatant (10 μL) was injected into the HPLC system for analysis.
Results
Optimization of Derivatization Conditions:
- Acid Selection: Trichloroacetic acid (1.0 mol/L) produced the highest peak area and was selected for further experiments.
- Trichloroacetic Acid Volume: The optimal volume was 100 μL, providing the maximum peak area.
- Reaction Temperature: The peak area increased with temperature up to 80 °C, beyond which decomposition of the derivative occurred. 80 °C was chosen as the optimal temperature.
- Reaction Time: A reaction time of 30 minutes gave the highest peak area.
- AHAD Concentration: The optimal volume of AHAD was 250 μL, as further increases did not improve the peak area and did not cause significant interference.
HPLC Separation:
- Column Selection: The ZORBAX SB-C18 column provided the best resolution (R = 1.9) for both standard and sample solutions at 30 °C.
- Retention Time: The MDA derivative had a retention time of 12.52 minutes under optimal chromatographic conditions (gradient elution using water and acetonitrile).
Stability of AHAD and MDA Derivative:
- AHAD Stability: AHAD remained stable at 4 °C for at least a week without affecting derivatization efficiency.
- MDA Derivative Stability: The MDA derivative showed less than 2.7% variation in peak area when stored at 4 °C for up to 48 hours, indicating high stability.
Selectivity:
- The method showed no interference from other aldehydes (formaldehyde, glyoxal, acrolein, and heptanal) in rat prostate samples, confirming good selectivity for MDA.
Linearity and Detection Limits:
- Linearity: The method showed a linear response for MDA in the range of 0.02 to 2.5 pmol with a correlation coefficient of 0.9998.
- LOD: The limit of detection (LOD) was 2.5 fmol (0.25 nmol/L).
- LOQ: The limit of quantification (LOQ) was 8.3 fmol (0.83 nmol/L).
Precision and Recovery:
- Instrumental Precision: RSD (%) for peak area at three concentrations (1.0, 5.0, and 25 nmol/mL) ranged from 0.69% to 1.06%.
- Analytical Precision: Intra-day precision ranged from 1.36% to 2.27%, and inter-day precision ranged from 2.36% to 3.92%.
- Recovery: The method achieved recoveries in the range of 97–102% with an RSD <4.7%.
Advantages of the Method:
Compared to other derivatization reagents like TBA and FMOC-hydrazine, AHAD provided higher sensitivity, a lower LOD (0.25 nmol/L), milder reaction conditions (80 °C), and a faster derivatization time (30 minutes), making it superior in terms of simplicity and efficiency.
Application:
The developed method was successfully applied to determine MDA levels in rat prostate samples, with satisfactory results. This is the first report of HPLC analysis of MDA in rat prostate, and the method can be adapted for other sample types.
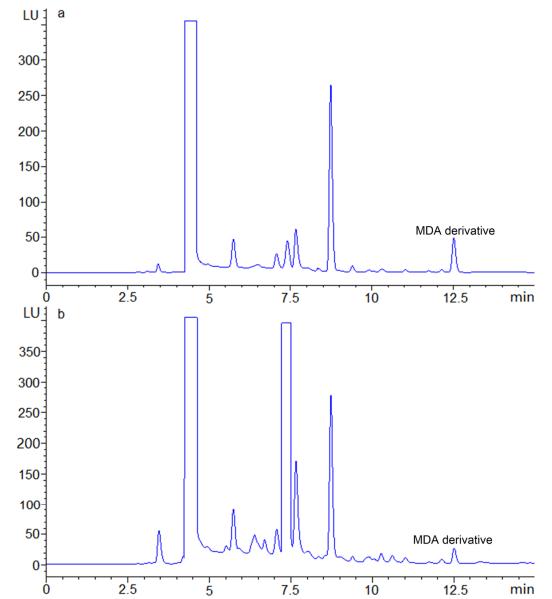 Chromatogram of standard MDA (a) and MDA in rat prostate sample
Chromatogram of standard MDA (a) and MDA in rat prostate sample
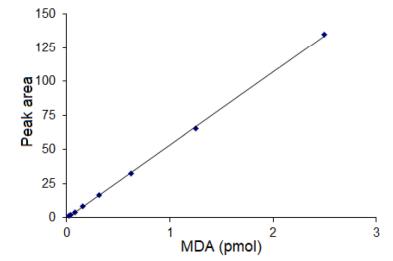 The calibration curve of MDA
The calibration curve of MDA
Reference
- Dong, Xiuli, Jiayuan Tang, and Xiangming Chen. "Sensitive determination of malondialdehyde in rat prostate by high performance liquid chromatography with fluorescence detection." Scientific Reports 10.1 (2020): 3990. https://doi.org/10.1038/s41598-020-61074-3




