Formic Acid and Formate Analysis | IC, LC–MS/MS & GC–MS
Volatile, reactive, and easy to mis-measure—formic acid and formate can skew QC, drive corrosion, and trigger compliance risk even at ppb.
Creative Proteomics delivers matrix-specific formic acid analysis and formate quantification using IC, LC-MS/MS, and headspace GC-MS, combining isotope dilution with rigorous QC so you can verify purity, trace contaminants, and control processes with confidence.
Why us?
- ppb-level LOQs with isotope-dilution accuracy for acid, formate, and alkyl formates
- Right-fit methods (IC | LC-MS/MS | HS-GC-MS) chosen to match matrix, range, and interferences
- Speciation you can trust—acid vs. formate agreement under pH-controlled workup
- Robust QC: blanks, spikes, duplicates, verification standards; clear uncertainty near decision limits
- Route markers & interferents reported on request (methanol, formaldehyde, glycolic/glyoxylic, acetate, etc.)
Submit Your Request Now
×
- What We Provide
- Advantages
- Technology Platform
- Sample Requirements
- Demo
- FAQs
What is Formic Acid?
Formic acid, also known as methanoic acid, is the simplest member of the carboxylic acid family. It is a colorless, highly polar liquid with a strong, pungent odor and a tendency to dissociate in water into the formate ion, depending on pH and ionic conditions.
Naturally, formic acid is produced by insects, plants, and microbial fermentation, but it is also a common by-product in chemical manufacturing, solvent oxidation, and atmospheric reactions. Because of its volatility and reactivity, even trace amounts can influence product quality, corrosion behavior, and environmental safety.
Accurate quantification of formic acid and its related species is therefore critical for process control, contamination tracing, and compliance testing in pharmaceutical, chemical, food, and environmental applications.
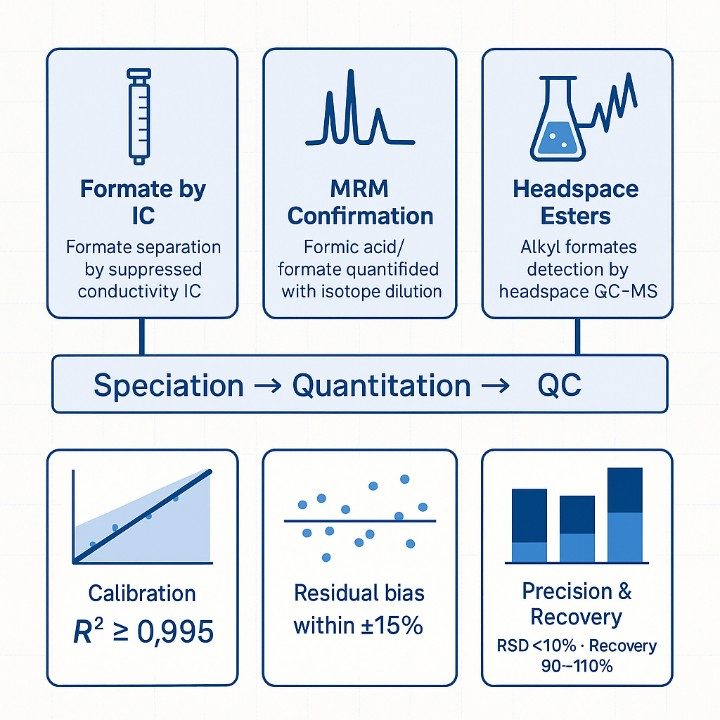
Service Scope: Formic Acid Quantification & Profiling
Creative Proteomics provides a modular and matrix-specific approach to formic acid testing. Depending on your sample type and analytical goals, we offer the following services:
- Quantitative analysis of free formic acid in aqueous, organic, or biological matrices.
- Formate ion (HCOO⁻) determination in process water, buffer systems, and formulation media.
- Volatile ester profiling, including methyl, ethyl, and propyl formate, for odor, residue, or migration studies.
- Air and off-gas monitoring using sorbent tubes or canisters with derivatization and GC-based detection.
- Impurity tracking in raw materials, intermediates, and APIs to support process validation and QC.
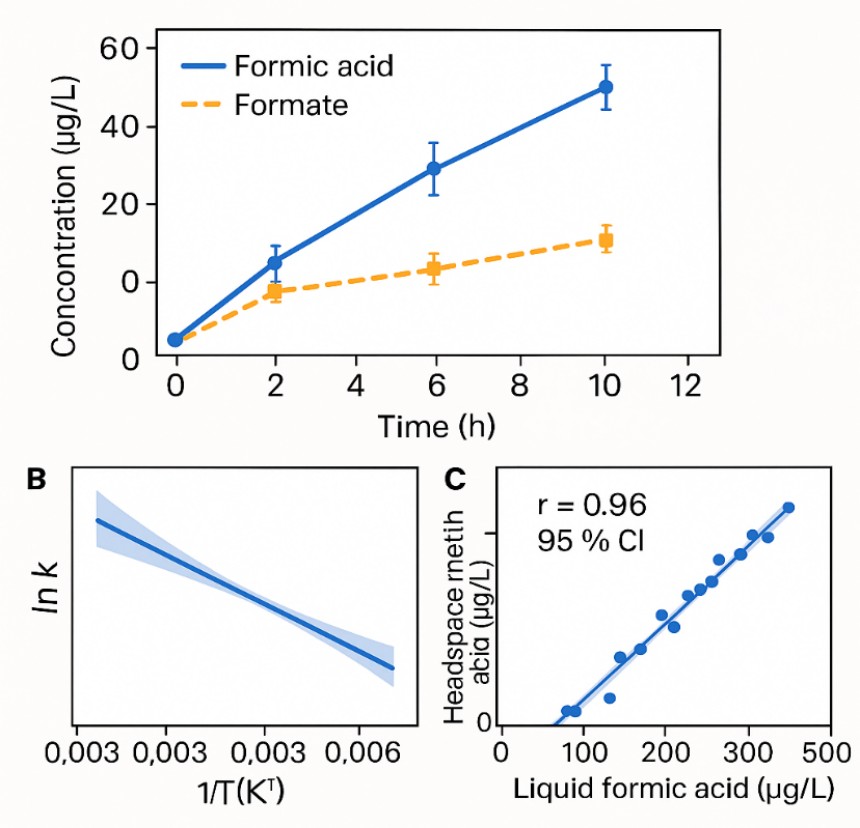
- Stability and degradation studies under pH, thermal, or oxidative stress to evaluate formic acid formation or loss.
Target Analytes in Our Formic Acid Panel
| Category | Target Analytes |
|---|---|
| Formic species (core) | Formic acid (HCOOH); Formate ion (HCOO⁻) |
| Inorganic/organic formate salts | Sodium formate; Potassium formate; Ammonium formate; Calcium formate; Magnesium formate; Lithium formate; Choline formate; Triethylammonium formate |
| C1–C2 alkyl formates | Methyl formate; Ethyl formate |
| C3–C5 alkyl/branched formates | n-Propyl formate; Isopropyl formate; n-Butyl formate; sec-Butyl formate; iso-Butyl formate; tert-Butyl formate; Isoamyl (isopentyl) formate |
| C6–C8 & aromatic formates | Hexyl formate; 2-Ethylhexyl formate; Benzyl formate |
| Unsaturated/functional formates | Allyl formate; Propargyl formate |
| Mechanistic/route markers (optional add-ons) | Methanol; Formaldehyde; Acetic acid; Glycolic acid; Glyoxylic acid; Oxalic acid |
| Common potential interferents (optional reporting) | Acetate; Lactate; Chloride; Sulfate; Nitrate |
Why Choose Our Formic Acid Analysis
- Typical LOQs: IC/formate 10–50 µg/L; LC-MS/MS 1–10 µg/L; headspace GC-MS 0.5–5 µg/L (water).
- Air monitoring achieves low-ppb v/v for common alkyl formates via headspace GC-MS.
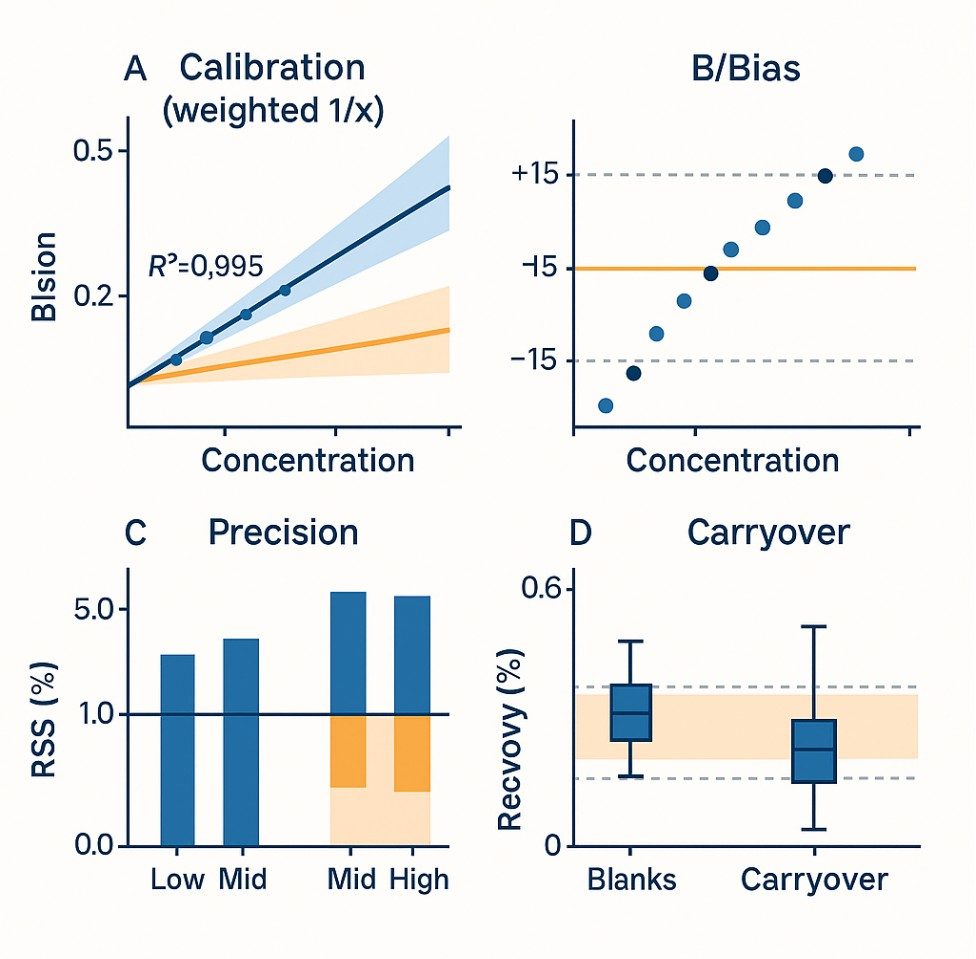
- Precision suited to QC: intra-batch 3–8% RSD; inter-batch 5–12% RSD.
- Spike recovery commonly 88–112% with matrix-matched calibration and isotope dilution.
- Linearity over ≥3 orders of magnitude (weighted fits, R² ≥ 0.995).
- Carryover controlled: typically<0.1% of high-standard response; blanks below LOQ.
- Reported uncertainty (k≈2) generally ±8–20% near decision limits.
- Speciation agreement (acid vs. formate) typically within ±10% under pH-controlled workup.
Formic Acid Analysis Workflow: Step-by-Step Process
- Scoping & method fit
Define matrix, targets, expected range, and reporting units. Select IC, LC-MS/MS, or headspace GC. - Sampling & stabilization
Specify containers, preservatives, and temperatures to prevent loss or conversion in transit. - Intake & QC planning
Log samples. Assign blanks, spikes, and duplicates with acceptance windows agreed in advance. - Preparation & cleanup
Perform dilution, filtration, pH control, or derivatization. Use isotope standards where applicable. - Instrument run
Acquire data under validated conditions. Include calibration, verification standards, and controls. - Quantitation & review
Apply matrix-matched calibration. Check recovery, precision, and blank status before release. - Reporting & interpretation
Deliver results, QC summaries, and method notes. Provide next-step guidance when requested.

Formic Acid Instrumentation & Platform Capabilities
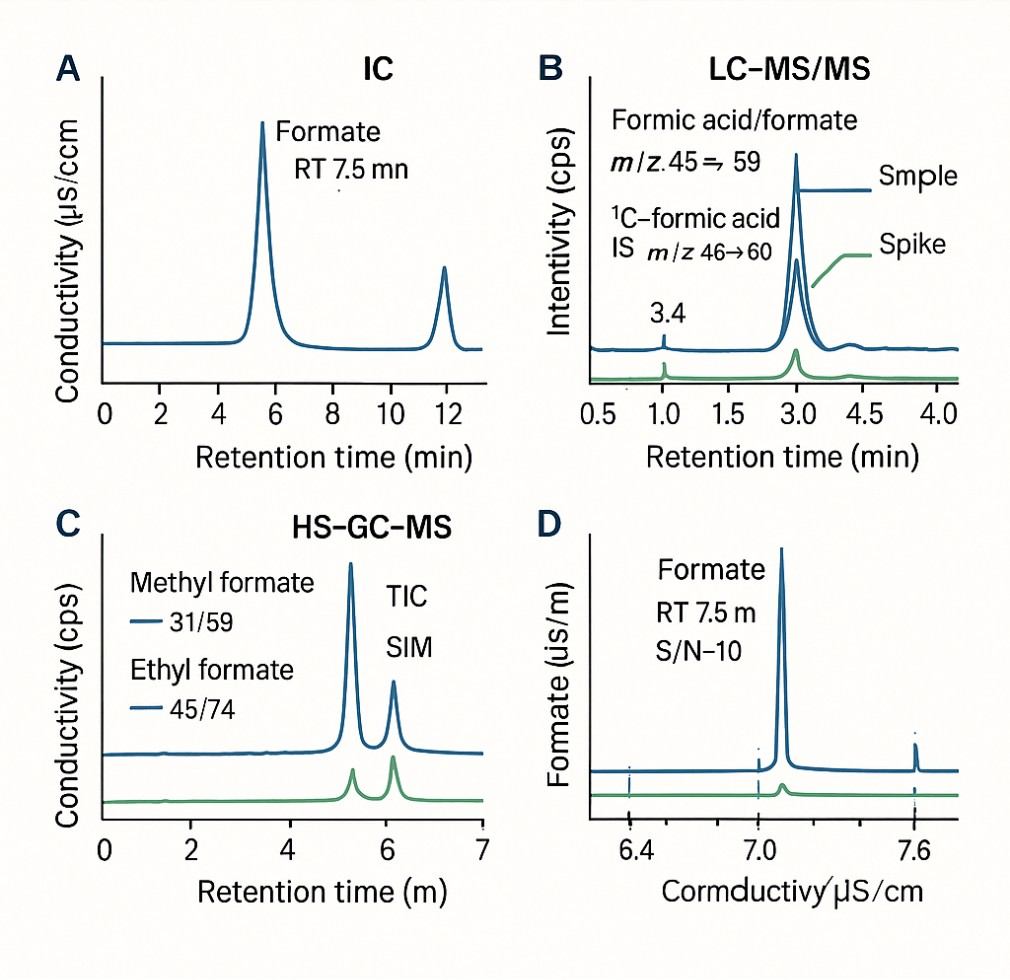
Ion Chromatography (IC)
System: Thermo ICS-6000 or equivalent
Detector: Suppressed conductivity
Columns: High-capacity anion-exchange with guard column
LOQ: ~10–50 µg/L (formate)
Application: Clean aqueous, buffer, and high-salt matrices
LC–MS/MS
System: Sciex QTRAP
Ionization: ESI−
Mode: Targeted MRM with isotope dilution (13C-formic acid)
LOQ: ~1–10 µg/L (formic acid/formate)
Application: Biological fluids, formulations, degraded samples
GC–FID / GC–MS
System: Agilent 7890 GC + 5977B MS
Column: DB-WAX or equivalent polar phase
LOQ: ~0.5–5 µg/L in liquids; ppb–ppm v/v in air
Application: Methyl/ethyl formate; derivatized formic acid; off-gas and solvent residue
Quality Controls
Matrix-matched calibration
Calibration verification standard (every 10–15 samples)
Method blanks, spiked matrix, and duplicate samples
Precision, recovery, and uncertainty reported with all datasets

Thermo ICS-6000 (Figure from Thermo)

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Agilent 7890B-5977A (Figure from Agilent)
Sample Requirements and Handling Guidelines for Formic Acid Assay
| Sample Type | Minimum Volume / Amount | Preferred Container | Preservation & Storage | Notes |
|---|---|---|---|---|
| Aqueous solutions / process water | ≥5 mL | HDPE or PP vial, airtight | Refrigerate at 2–8 °C; avoid glass for low-level formate | Analyze promptly to minimize microbial conversion |
| Organic solvents (e.g., MeOH, IPA) | ≥5 mL | Amber glass with PTFE-lined cap | Store cold, minimize headspace, protect from light | Suitable for volatile esters or trace acid impurity |
| Biological fluids (e.g., plasma, urine) | ≥200 µL | Cryovial, polypropylene | Freeze at −20 °C or lower; avoid repeated freeze–thaw | Use labeled, low-binding tubes where possible |
| Pharmaceutical formulations | ≥200 mg or 0.5 mL | Amber glass vial or tube | Store as per formulation guidelines; avoid exposure to air | Include excipient list for matrix assessment |
| Solid raw materials / powders | ≥200 mg | Amber glass jar, tightly sealed | Store cool and dry; minimize air/moisture exposure | Grind or homogenize if heterogeneous |
| Cell culture media / fermentation broth | ≥5 mL | Sterile plastic tube or HDPE bottle | Chill immediately; avoid CO₂ exposure for pH-sensitive media | Include medium composition or pH if known |
Note: If your matrix type is not listed, or if volume is limited, please contact us for a tailored plan. We offer low-volume workflows and pilot testing where needed.
Demo Results
FAQ of Formic Acid Analysis
Do I need to specify whether I need formic acid or formate tested?
Not necessarily. If the sample is aqueous, pH-dependent speciation will be considered during method selection. We can quantify total formic species or report acid and formate separately if needed.
What if my sample contains both formic acid and volatile esters like methyl formate?
We can distinguish and quantify both using a combination of LC–MS/MS and headspace GC–MS. These species are chemically distinct and often require separate workflows.
How stable is formic acid in biological or buffered samples?
Formic acid can degrade or convert depending on pH, temperature, and microbial content. Freezing or cold storage is strongly recommended, and preservatives may be suggested for specific matrices.
Can high salt or buffer content interfere with formate analysis?
Yes, especially for IC. High-ionic-strength matrices can suppress or overlap signals. We adjust dilution, column chemistry, or switch to LC–MS/MS when interference is expected.
How are detection limits affected by complex matrices like plasma or formulations?
LOQs may be higher in complex matrices due to background noise or ion suppression. We apply matrix-matched calibration and internal standards to maintain quantitation integrity.
Is headspace GC–MS sensitive enough for low-level formate esters in packaging or air?
Yes. Typical LOQs for methyl or ethyl formate in air or headspace are in the low-ppb range, sufficient for trace-level screening or migration studies.
Do I need to submit standards or controls with my samples?
No, unless you require custom calibration. We maintain validated internal standards and can source certified reference materials as needed.
What information should I include with the sample submission?
Please provide matrix type, sample volume, expected concentration range (if known), and whether speciation or total quantification is required. This ensures proper method assignment and accurate reporting.
Can you test for formic acid in the presence of other small acids like acetic or glycolic acid?
Yes. Our methods are validated to separate and quantify structurally similar acids, and platform selection (IC vs. LC–MS/MS) is based on matrix composition and required specificity.
Learn about other Q&A about proteomics technology.
Publications
Here are some of the metabolomics-related papers published by our clients:

- Proteolytic activation of fatty acid synthase signals pan-stress resolution. Nature Metabolism.
- Prospective randomized, double-blind, placebo-controlled study of a standardized oral pomegranate extract on the gut microbiome and short-chain fatty acids. Foods.
- Calcium homeostasis and stable fatty acid composition underpin heatwave tolerance of the keystone polychaete Hediste diversicolor. Environmental Research.
- B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nature communications.