The strength and mode of intermolecular interactions are important indicators for evaluating candidate drugs in drug development. There are many methods for measuring the interaction strength between drug molecules and targets, including NMR (nuclear magnetic resonance), MST (microthermal mobility), ITC (isothermal titration calorimetry), fluorescence polarization and SPR (Surface plasmon resonance), which is essentially a physical optical phenomenon.
What is SPR and Its Principle
SPR is a phenomenon where the oscillation of electrons on a metal surface is excited by incident light. This leads to the creation of a resonant wave known as a surface plasmon.
SPR occurs at the interface between a metal and a dielectric material and is highly sensitive to changes in the refractive index of the dielectric layer, allowing it to be used as a sensing technique. In an SPR setup, light is directed at a metal film, such as gold or silver, that is covered with a thin dielectric layer. The incident light excites the electrons on the metal surface into a resonant wave. The angle at which this wave is created is related to the refractive index of the dielectric layer and changes in this refractive index result in changes in the angle at which the wave is created. This shift in angle can be detected by analyzing the reflected light from the metal surface.
SPR is widely used in biosensing applications due to its high sensitivity, real-time measurement capability, and non-invasive nature. For example, it can be used to study the interaction between a molecule and a solid surface, monitor protein-protein interactions, and detect small changes in the concentration of biomolecules in solution.
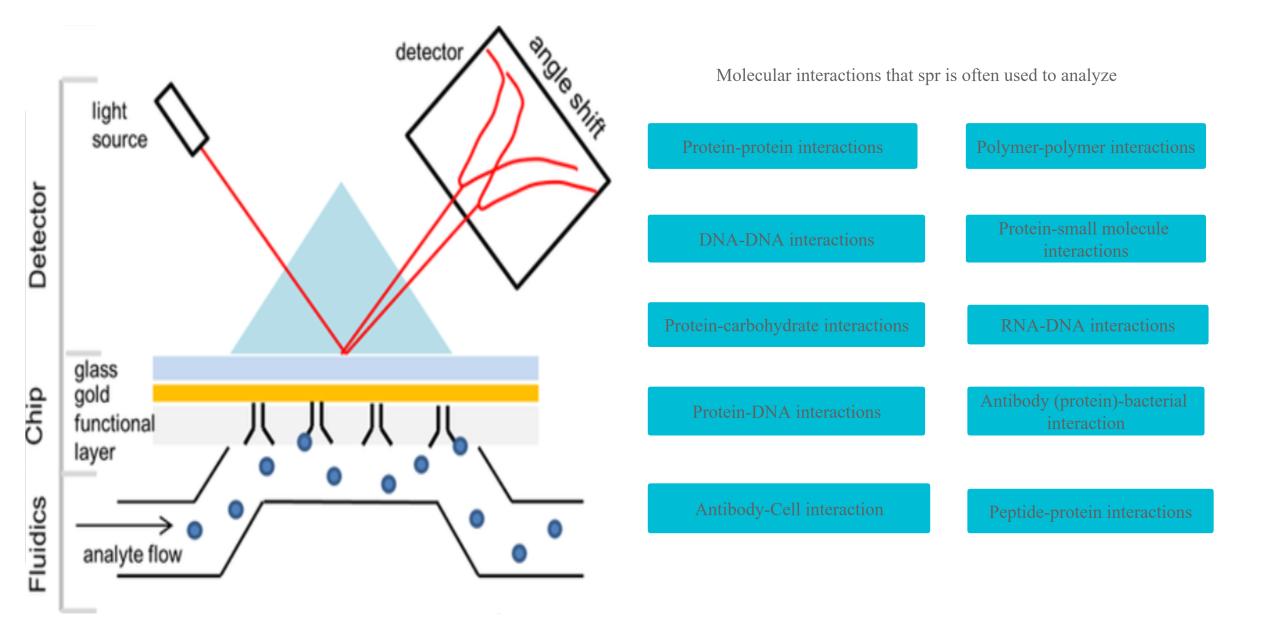
Application of SPR Assay
SPR assay is a widely used technique in the biological field due to its high sensitivity and real-time measurement capability. Some of the key applications of SPR in the biological field are:
Studying molecular interactions: SPR can be used to study the interaction between two or more molecules, such as protein-protein interactions, protein-DNA interactions, and protein-ligand interactions.
Detection of small molecules: SPR is sensitive enough to detect small changes in the concentration of biomolecules in solution, making it a useful tool for quantifying the presence of small molecules, such as drug candidates or metabolites, in biological samples.
Kinetics analysis: SPR can be used to determine the kinetics of molecular interactions, such as the association and dissociation rates of two interacting molecules, by analyzing the binding curves obtained in real-time.
Characterization of antibodies: SPR can be used to determine the binding affinity and specificity of antibodies for their target antigens. This is useful for the development of diagnostic assays, as well as for the quality control of therapeutic antibodies.
Biosensors: SPR can be integrated into biosensors, which use biological molecules such as enzymes or antibodies to detect the presence of specific analytes in a sample.
The Advantages of the SPR Assay
Real-Time Analysis: SPR assays provide real-time information about molecular interactions, including association and dissociation kinetics, affinity, and specificity. This allows for the rapid screening of potential binders and the optimization of binding conditions.
High Sensitivity: SPR assays are extremely sensitive, with a detection limit in the picomolar to nanomolar range. This sensitivity makes it possible to detect weak or low-affinity interactions, making it ideal for the study of biomolecular interactions.
Label-free Detection: SPR assays are label-free, meaning they do not require any fluorescent or radioactive labels to be added to the sample. This eliminates the need for time-consuming labeling procedures and reduces the risk of interference with the biological activity of the sample.
Quantitative Analysis: SPR assays provide quantitative information about molecular interactions, including binding constants, dissociation rates, and concentration information. This information can be used to optimize binding conditions and to rank binders.
Multiplex Analysis: SPR assays can be used to analyze multiple interactions simultaneously, allowing for high-throughput screening of large libraries of potential binders. This makes it possible to identify the best binders in a single experiment, reducing the time and cost associated with multiple independent assays.
Versatility: SPR assays can be used to study a wide range of molecular interactions, including protein-protein, protein-DNA, protein-small molecule, and antibody-antigen interactions. This versatility makes it an indispensable tool for the study of biological systems.
The SPR assay is a valuable tool for the analysis of molecular interactions due to its high sensitivity, real-time analysis, label-free detection, quantitative information, multiplex analysis, and versatility.






