Corticosterone Analysis Service
Corticosterone is a key biomarker of stress, endocrine regulation, and pharmacological response—especially in preclinical models. At Creative Proteomics, we deliver ultra-sensitive, LC-MS/MS-based corticosterone analysis designed for accuracy, matrix versatility, and robust biological interpretation. Empower your research with hormone data you can trust.
Why Choose Creative Proteomics?
- LC-MS/MS detection down to 0.02 ng/mL
- Simultaneous profiling of corticosterone, cortisol, and precursors
- Validated for plasma, serum, saliva, CSF, tissues
- Matrix-specific extraction with isotope-labeled internal standards
- QC ensured: Recovery ≥90%, CV ≤10%, R² ≥0.995
Submit Your Request Now
×
What You Receive
- Raw & processed LC-MS/MS data (PDF + Excel)
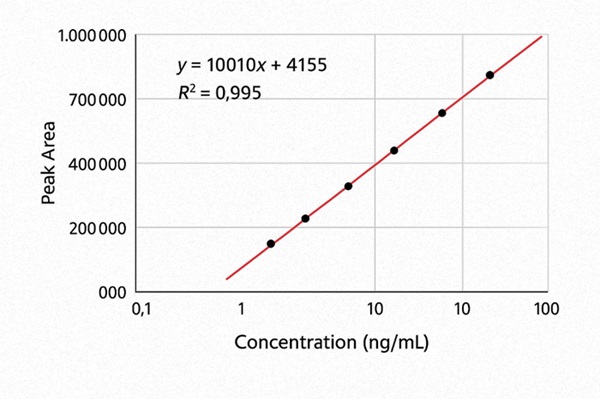
- 7-point calibration curves with R²
- QC recovery & CV metrics
- ng/mL or ng/g concentration tables
- Optional: hormone trend plots, PCA heatmaps
- Why Choose Us
- What We Provide
- Technology Platform
- Sample Requirement
- Demo
- FAQs
What is Corticosterone?
Corticosterone is a pivotal glucocorticoid hormone synthesized in the adrenal cortex, primarily involved in the regulation of energy metabolism, immune responses, and adaptation to environmental stressors. While its physiological relevance is particularly prominent in rodent and avian models (serving as the functional equivalent of cortisol in humans), corticosterone also plays critical roles across species in mediating hypothalamic-pituitary-adrenal (HPA) axis activity.
In preclinical, pharmacological, and behavioral studies, precise quantification of corticosterone serves as a key biomarker for assessing stress responses, circadian hormone fluctuations, drug efficacy, and neuroendocrine dynamics.
Why Choose Creative Proteomics for Corticosterone Analysis?
Creative Proteomics delivers specialized corticosterone detection solutions using high-sensitivity LC-MS/MS platforms, enabling ultra-precise, reproducible, and multiplexed steroid profiling across diverse biological matrices.
Our service is tailored to meet the needs of:
- Preclinical research teams investigating HPA-axis dynamics or drug-induced endocrine modulation
- Behavioral and neurological scientists exploring hormone-behavior interactions
- Pharmacologists studying glucocorticoid-mediated mechanisms or developing corticosteroid-based therapeutics
- Toxicologists monitoring stress hormone shifts in environmental or exposure studies
Key Advantages:
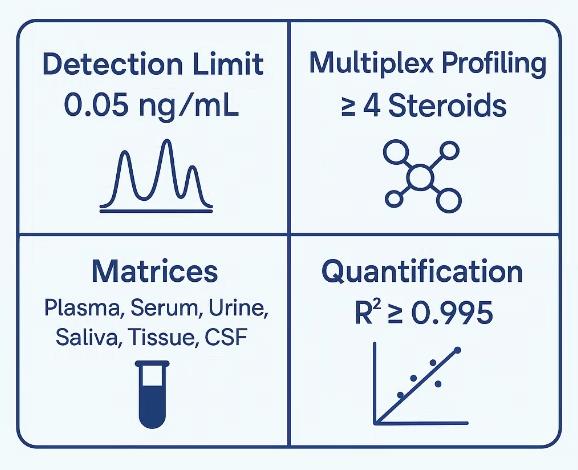
- Ultrasensitive Detection Limit: As low as 0.05 ng/mL in plasma or serum
- Matrix Versatility: Compatible with plasma, serum, urine, saliva, cerebrospinal fluid (CSF), tissues, and culture media
- Isotope-Labeled Internal Standards: Ensures quantification accuracy and inter-sample consistency
- Multiplex Profiling: Simultaneous detection of corticosterone, cortisol, cortisone, and related glucocorticoids
- Comprehensive QC: Recovery rate ≥90%, CV ≤10%, linearity R² ≥ 0.995
What Research Challenges Does This Service Solve?
| Research Challenge | How This Service Helps |
|---|---|
| Low abundance of corticosterone in peripheral fluids | High-sensitivity LC-MS/MS quantification with sub-ng/mL resolution |
| Matrix interference in hormone detection | Matrix-specific sample preparation protocols and SPE purification |
| Dynamic hormone fluctuations under stress or time-course | Fast, reproducible throughput to support high-frequency sampling or circadian studies |
| Cross-reactivity in immunoassays (e.g., ELISA limitations) | Specific MRM transitions to eliminate false positives and enhance specificity |
| Multihormonal assessment needs | Multiplex panels covering glucocorticoids, mineralocorticoids, and precursors |
Our Corticosterone Analysis Services
Creative Proteomics offers a suite of customizable analysis services tailored to your study model and research goals:
High-Sensitivity LC-MS/MS Quantification
Accurate detection of corticosterone down to sub-ng/mL levels using stable isotope-labeled internal standards and robust calibration. Suitable for plasma, serum, saliva, tissue, and other complex matrices.
Multiplex Glucocorticoid Profiling
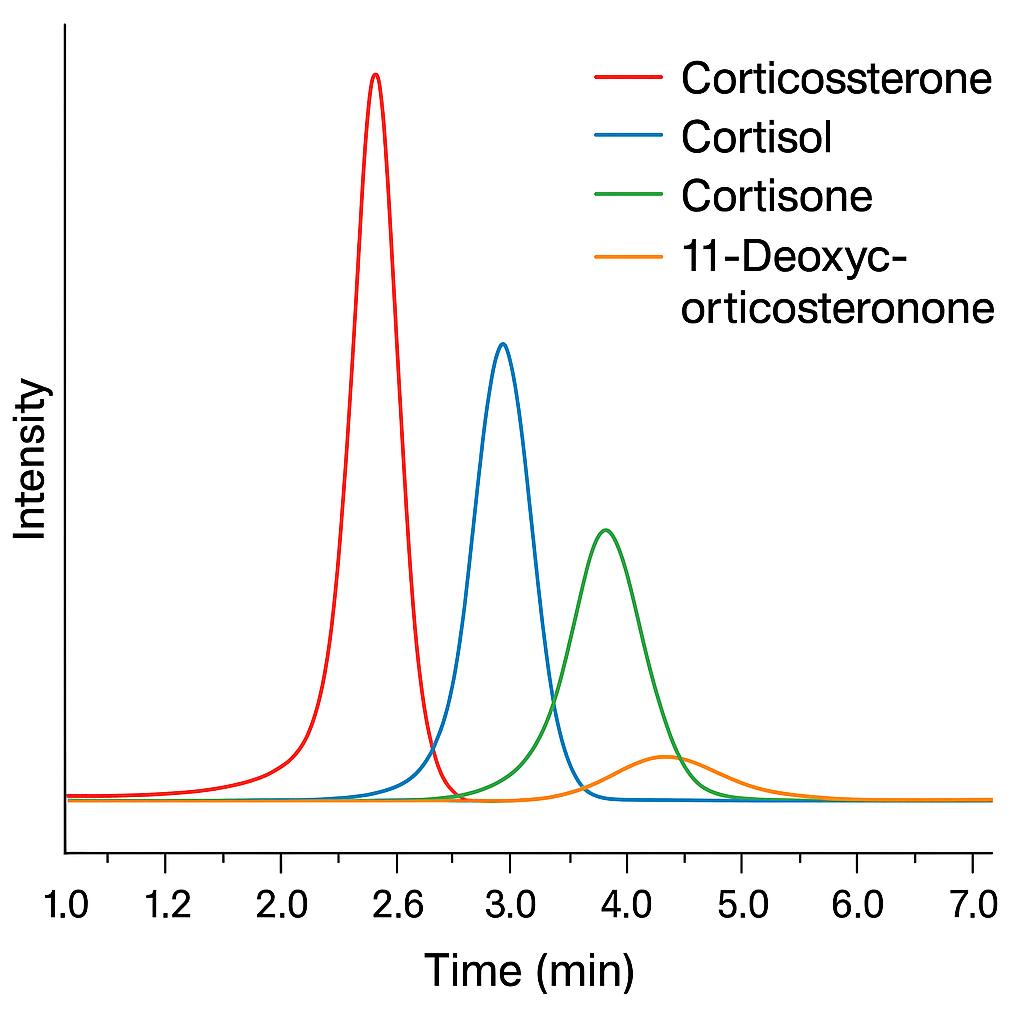
Simultaneous quantification of corticosterone, cortisol, cortisone, and 11-deoxycorticosterone in a single run. Ideal for evaluating upstream and downstream hormone dynamics within the adrenal steroidogenesis pathway.
Optimized Sample Preparation for Diverse Matrices
Matrix-specific workflows including protein precipitation, liquid-liquid extraction (LLE), and solid-phase extraction (SPE) ensure high recovery and minimal interference across sample types such as brain, adrenal tissue, CSF, and cell culture supernatants.
Time-Course and Pharmacodynamic Studies
Support for longitudinal hormone monitoring with batch-consistent analysis and flexible sample batching. Particularly suited for circadian rhythm studies, stress modeling, and endocrine intervention assessments.
List of Detected Corticosterone and Related Steroid Analytes
| Analyte Name | Steroid Class | Biological Significance |
|---|---|---|
| Corticosterone | Glucocorticoid | Primary glucocorticoid in rodents and birds; central to stress response |
| Cortisol | Glucocorticoid | Human primary stress hormone; useful for cross-species comparisons |
| Cortisone | Glucocorticoid (inactive) | Inactive metabolite of cortisol; reflects cortisol turnover |
| 11-Deoxycorticosterone | Mineralocorticoid precursor | Intermediate in corticosterone synthesis pathway |
| 11-Deoxycortisol | Glucocorticoid precursor | Key precursor; used to assess enzymatic activity in adrenal biosynthesis |
| 17α-Hydroxyprogesterone | Steroid precursor | Intermediate in both glucocorticoid and sex steroid biosynthesis |
| Progesterone | Steroid precursor | Upstream precursor for corticosterone, aldosterone, and androgens |
| Aldosterone (optional) | Mineralocorticoid | Involved in sodium/potassium balance; co-detected in HPA axis profiling |
| Testosterone (optional) | Androgen | For integrated studies linking stress and reproductive hormones |
| DHEA (optional) | Steroid precursor | Links adrenal biosynthesis to neurosteroid and androgen pathways |
Note: Optional targets can be included in customized panels upon request. Additional glucocorticoid metabolites or species-specific variants can be validated on demand.
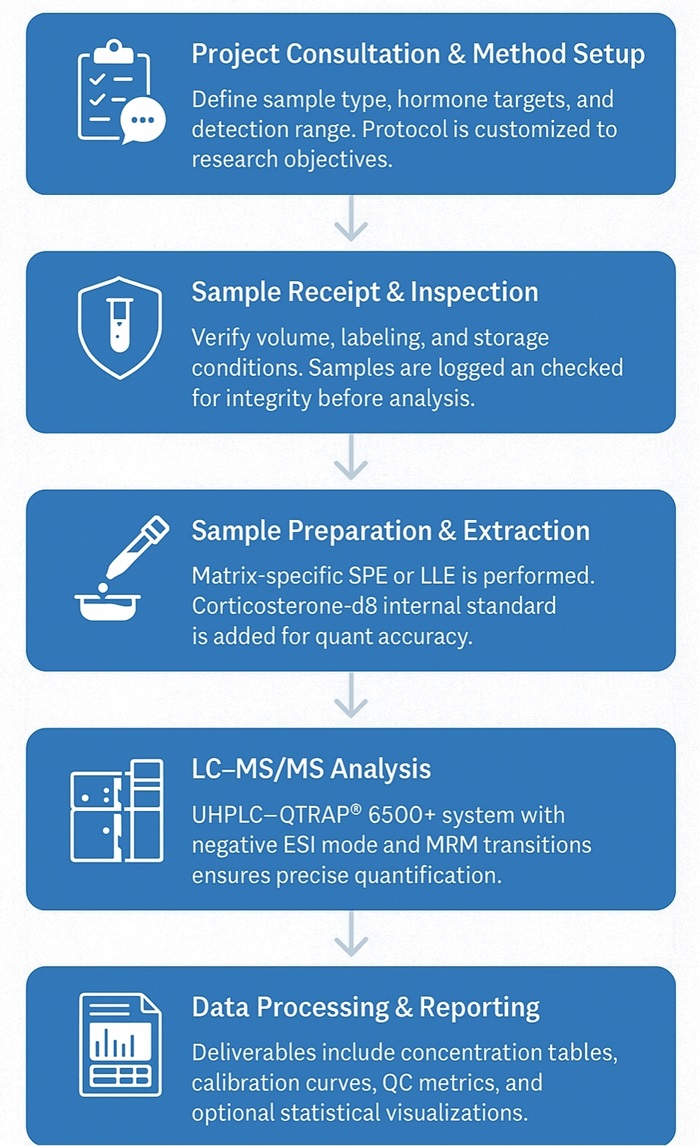
Workflow for Corticosterone Analysis Service
1. Project Consultation & Method Customization
We start by understanding your study design, sample type, and target detection range. Based on your research goals, we provide tailored protocol recommendations and detection panel options.
2. Sample Receipt & Quality Check
Each sample is verified for volume, labeling, and storage conditions upon arrival. Samples are logged into our LIMS system for full traceability. Visual inspection and integrity checks are performed to ensure sample quality.
3. Sample Preparation & Extraction
Biological matrices undergo optimized solid-phase extraction (SPE) or liquid-liquid extraction (LLE) with antioxidant stabilization. Stable isotope-labeled internal standards (e.g., corticosterone-d8) are added to correct for recovery and matrix variability.
4. LC-MS/MS Quantification
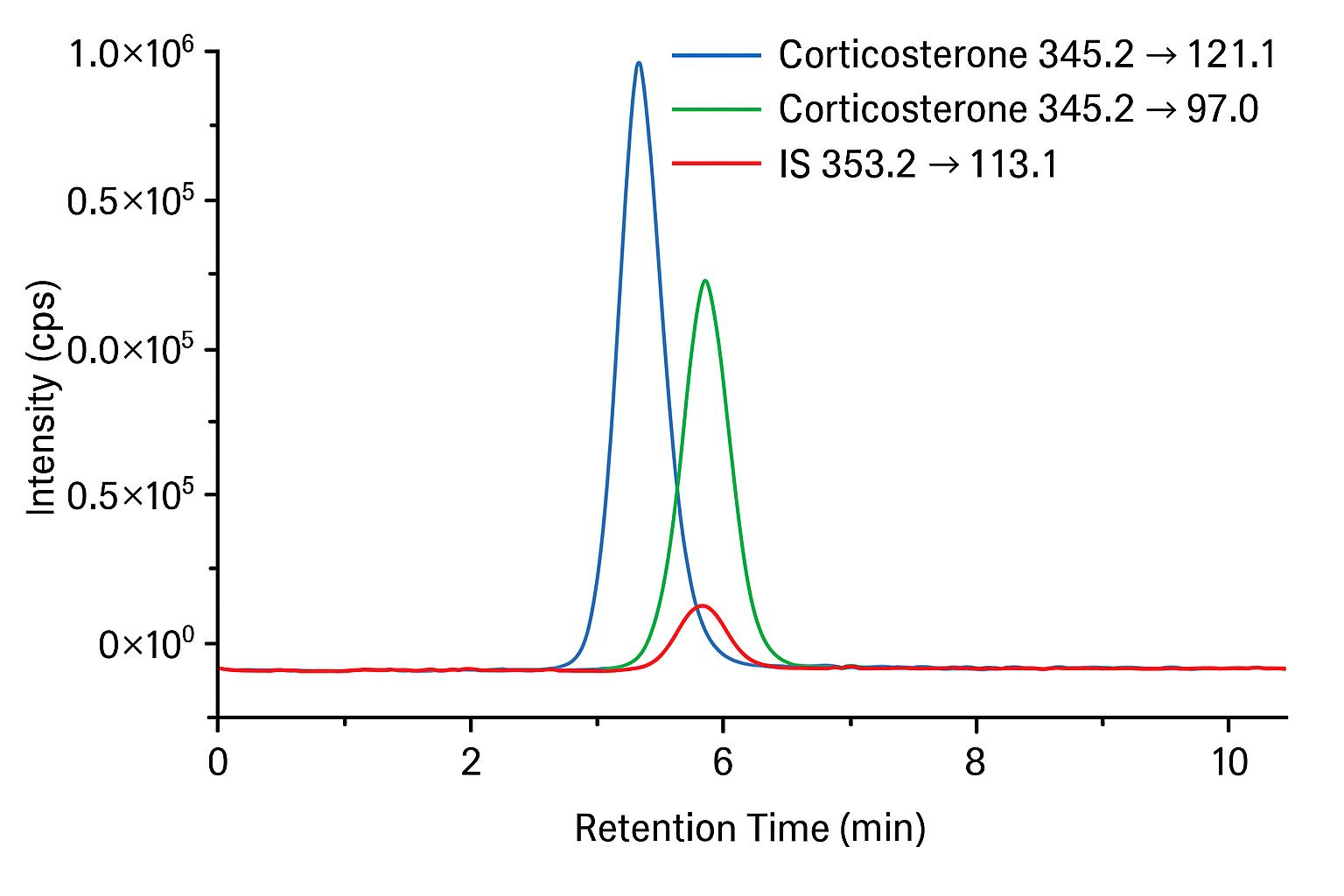
Quantitative analysis is performed using UHPLC–QTRAP® 6500+ triple quadrupole mass spectrometry in negative ESI mode. A 7-point calibration curve ensures linearity, and both quantifier and qualifier MRM transitions are used for accurate identification.
5. Data Analysis & Report Delivery
Final results include raw and processed data, calibration curves, recovery rates, and concentration tables (ng/mL or ng/g). Optional statistical analysis, fold-change plots, or hormone trend visualizations are available upon request.
Technology Platform for Corticosterone Analysis Service
Instrument: AB Sciex QTRAP® 6500+ Triple Quadrupole LC-MS/MS System
Chromatography: Ultra-high-performance liquid chromatography (UHPLC) using Agilent 1290 Infinity II System
Column: Waters ACQUITY UPLC BEH C18 (2.1 × 50 mm, 1.7 μm particle size)
Mobile Phase: Gradient elution with water (0.1% formic acid) and acetonitrile (0.1% formic acid)
Flow Rate: 0.3 mL/min
Injection Volume: 5 µL
Ionization Mode: Negative electrospray ionization (ESI−)
MRM Transitions:
- Corticosterone: m/z 345.2 → 121.1 (quantifier)
- Corticosterone: m/z 345.2 → 97.0 (qualifier)
- Internal standard (e.g., corticosterone-d8): m/z 353.2 → 125.1
Limit of Detection (LOD): ≤ 0.02 ng/mL
Limit of Quantification (LOQ): ≤ 0.05 ng/mL
Calibration Range: 0.05 ng/mL to 100 ng/mL with linearity R² ≥ 0.995
 SCIEX Triple Quad™ 6500+ (Figure from Sciex)
SCIEX Triple Quad™ 6500+ (Figure from Sciex)
 Waters ACQUITY UPLC System (Figure from Waters)
Waters ACQUITY UPLC System (Figure from Waters)
Sample Requirements for Corticosterone Analysis Service
| Sample Type | Recommended Volume | Storage Conditions | Notes |
|---|---|---|---|
| Plasma | ≥100 µL | -80°C | Use EDTA or heparin anticoagulant; avoid hemolysis |
| Serum | ≥100 µL | -80°C | Allow clotting at room temperature, centrifuge, and freeze promptly |
| Saliva | ≥200 µL | -80°C | Collect using steroid-free collection devices; avoid eating/drinking before sampling |
| Urine | ≥500 µL | -80°C | Prefer first-morning sample; avoid preservatives unless specified |
| Tissue (e.g., brain, adrenal) | ≥50 mg | -80°C | Flash freeze in liquid nitrogen; avoid repeated freeze-thaw cycles |
| Cell Culture Supernatant | ≥500 µL | -80°C | Use serum-free media if possible; pre-clear by centrifugation |
| Cerebrospinal Fluid (CSF) | ≥50 µL | -80°C | Requires high-sensitivity detection; handle with extreme care |
Demo Results
FAQ of Corticosterone Analysis Service
Can you analyze corticosterone in non-traditional or rare sample types?
Yes. In addition to commonly used matrices, we can validate the method for rare or unconventional samples such as fecal extracts, feathers (for avian studies), dried blood spots (DBS), or microdialysates upon request.
Is your method validated for both free and total corticosterone?
Yes. We can differentiate between free (unbound) corticosterone and total corticosterone by including a protein-binding disruption step during sample preparation if required.
Can you compare corticosterone levels across different species in the same study?
Absolutely. Our LC-MS/MS method is species-independent, allowing direct comparison of hormone concentrations across rodents, birds, reptiles, and other vertebrates without cross-reactivity issues.
Do you provide reference ranges or baseline data for interpretation?
While baseline levels vary widely by species, age, and experimental conditions, we can provide literature-based reference data or compile internal reference distributions from prior validated studies to assist interpretation.
Can you detect circadian rhythm-related fluctuations in corticosterone?
Yes. The method's high precision supports time-course sampling, enabling detection of subtle circadian or ultradian fluctuations in hormone levels.
Is it possible to integrate corticosterone analysis with other metabolic or endocrine endpoints?
Yes. We can design custom LC-MS/MS panels that include corticosterone alongside other glucocorticoids, mineralocorticoids, sex steroids, or selected metabolites, facilitating a more holistic interpretation of HPA axis activity.
How do you ensure comparability of results across large longitudinal or multi-site studies?
We maintain strict QC procedures, use stable isotope-labeled internal standards, and apply consistent calibration and instrument settings across batches to ensure inter-study comparability.
Can you process samples with limited volume or low concentration?
Yes. Our method requires as little as 50 μL for plasma or serum, and the optimized extraction protocol ensures reliable quantification even at sub-ng/mL levels.
Can you analyze corticosterone in combination with stress challenge or pharmacological intervention experiments?
Yes. Our platform supports both acute and chronic intervention studies, enabling quantitative assessment of hormone shifts in response to stress paradigms, drug administration, or environmental changes.
Do you offer blinded sample analysis for unbiased results?
Yes. We can process samples under blinded coding, ensuring unbiased data generation for clinical-style or preclinical research designs.
Learn about other Q&A about proteomics technology.
Publications
Here are some of the metabolomics-related papers published by our clients:

- A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B. 2023.
- The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. 2023.
- Lipid droplet-associated lncRNA LIPTER preserves cardiac lipid metabolism. 2023.
- Inflammation primes the kidney for recovery by activating AZIN1 A-to-I editing. 2023.
- Anti-inflammatory activity of black soldier fly oil associated with modulation of TLR signaling: A metabolomic approach. 2023.
- Non-invasive elevation of circulating corticosterone increases the rejection of foreign eggs in female American robins (Turdus migratorius). 2022.
- Untargeted metabolomics reveal sex-specific and non-specific redox-modulating metabolites in kidneys following binge drinking. 2023.
- Nicotine exposure during rodent pregnancy alters the composition of maternal gut microbiota and abundance of maternal and amniotic short chain fatty acids. 2022.
- Sex hormones, sex chromosomes, and microbiota: identification of Akkermansia muciniphila as an estrogen-responsive bacterium. 2023.