Title: Effects of polyol excipient stability during storage and use on the quality of biopharmaceutical formulations
Journal: Journal of Pharmaceutical Analysis
Published: Volume 12, Issue 5, October 2022, Pages 774-782
Background
Biopharmaceuticals often require excipients, like polyols, to stabilize their formulations. However, these excipients can degrade during storage or repeated use, generating impurities that negatively affect the quality of the biopharmaceuticals. This study specifically examines glycerol, a commonly used polyol excipient, and investigates its degradation and impact on a model biopharmaceutical, thymopentin. The research highlights the potential degradation of glycerol to unexpected impurities and the resulting instability of thymopentin. In contrast, another polyol, mannitol, was found to be more stable. The findings emphasize the need for manufacturers to monitor excipient quality, especially during long-term storage, to prevent degradation and ensure the stability of biopharmaceutical formulations.
Materials & Methods
Sample Preparation
- Thymopentin (1 mg/mL) in sodium phosphate buffer (pH 7.0, 10 mM) with EDTA.
- Mannitol and glycerol (undegraded/degraded) as excipients, filtered and aseptically filled into vials.
Accelerated Stability Study
- Excipients stored at 40°C for 6 weeks, vortexed biweekly.
- Formulations analyzed at 0, 1, 2, 4, and 8 weeks via RP-HPLC.
Detection of Aldehydes/Reducing Substances
- Phenol reagent spectrophotometry at 655 nm to detect aldehydes in excipients and formulations.
GC-MS
- Impurities in glycerol detected by GC-TOF-MS with Wax column, ionization in EI mode (35–450 amu).
RP-HPLC
- Supersil ODS column, gradient elution with 0.1% formic acid in water/acetonitrile.
- Thymopentin monitored at 214, 220, 254, and 280 nm.
LC-MS/MS
- LC on Agilent 1290 HPLC, MS on Agilent 6460 Triple Quad LC/MS.
- First-order scanning (50–1000 amu), tandem MS for degradation product confirmation.
Results
Color Reaction of Polyol Excipients:
Glycerol before degradation (G0) and mannitol (M0, M1) did not show a blue-green color, while degraded glycerol (G1) did, indicating the presence of aldehydes and reducing substances.
Impurities in Degraded Glycerol:
GC-MS identified several impurities in degraded glycerol, including hydroxyacetone, glycidol, and cyclic acetal/ketal derivatives. These were linked to oxidation reactions in glycerol and may affect the stability of biopharmaceuticals.
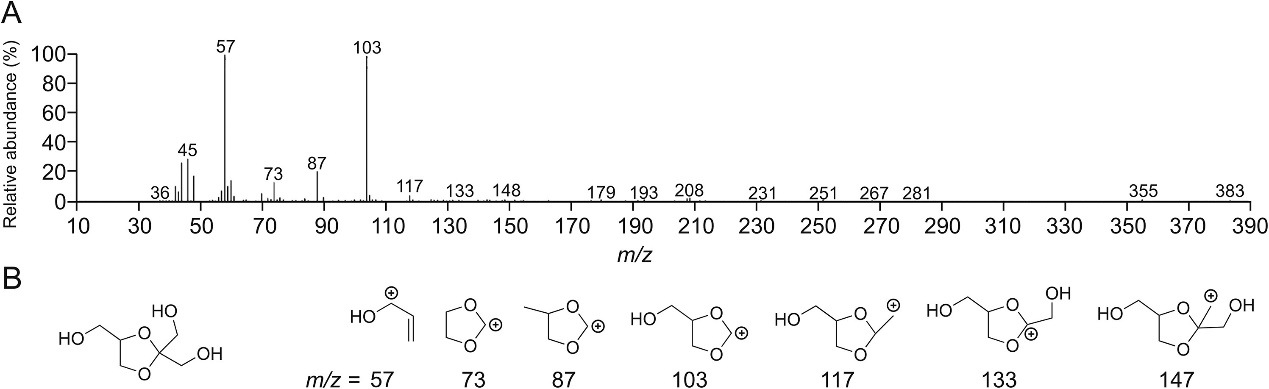 (A) Mass spectra of impurity 4 eluting at 7.4 min in gas chromatography with mass spectrometry (GC-MS). (B) The structure of impurity 4 and its mass spectrum fragments.
(A) Mass spectra of impurity 4 eluting at 7.4 min in gas chromatography with mass spectrometry (GC-MS). (B) The structure of impurity 4 and its mass spectrum fragments.
Stability of Thymopentin Formulations:
Accelerated stability tests showed that formulations with degraded glycerol (TP5-G1) had significantly lower thymopentin content and aldehyde levels compared to others, indicating that degraded glycerol promoted thymopentin degradation.
LC-MS/MS Analysis of Thymopentin Degradation:
Major degradation products of thymopentin were identified, including isoAsp-containing variants, and products formed through reactions with glycerol oxidation products (e.g., GLAD, DHA), confirming that aldehydes and ketones from glycerol degradation contributed to thymopentin instability.
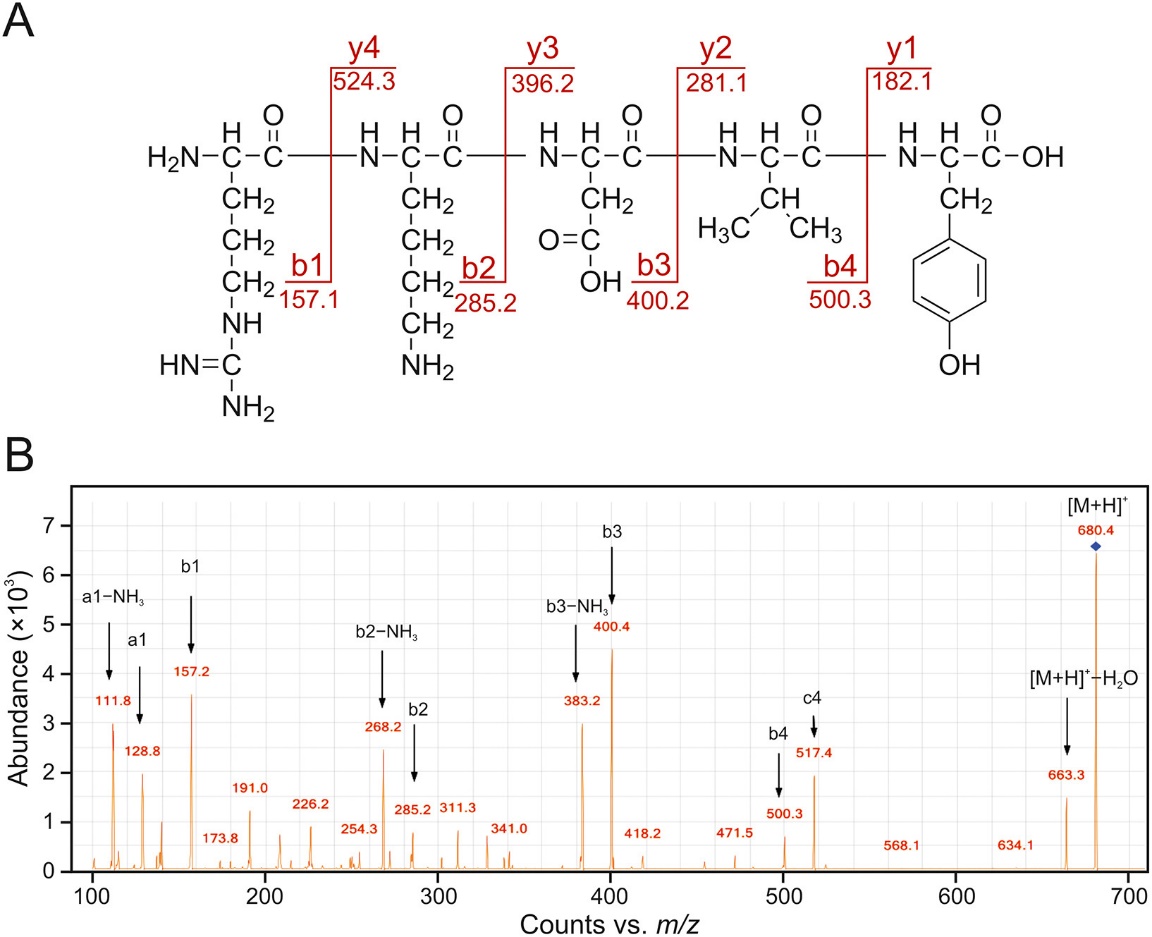 (A) MS/MS analysis of thymopentin as a representative. (B) MS/MS spectrum of thymopentin.
(A) MS/MS analysis of thymopentin as a representative. (B) MS/MS spectrum of thymopentin.
Reference
- Sun, Min-Fei, et al. "Effects of polyol excipient stability during storage and use on the quality of biopharmaceutical formulations." Journal of pharmaceutical analysis 12.5 (2022): 774-782. https://doi.org/10.1016/j.jpha.2022.03.003






