RNA Pull-Down Service — High-Confidence Mapping of RNA–Protein Interactions
Know who binds your RNA—and how strongly. Get defensible RBP hit lists with ≤1% FDR, replicate r ≥ 0.85, and wash-stringency validation, plus matched RNA–protein enrichment and pathway context.
- Purpose-built designs: custom baits/probes + mutant/competitor sets
- Fit-for-biology capture: native, UV254, or reversible formaldehyde
- Quant you can trust: DDA/DIA LC–MS/MS, 16+ plex multiplexing
- Controls baked-in: beads-only, scrambled, motif-mutant; cold-competition optional
- Transparent QC: RT drift ≤ ±90 s; QC peptide CV ≤ 15%; contaminant flags retained
Submit Your Request Now
×
- What We Provide
- Advantages
- Experimental Architecture
- Workflow
- Sample Requirement
- What You Will Receive
- FAQ
What Is RNA Pull-Down and When Should You Use It?
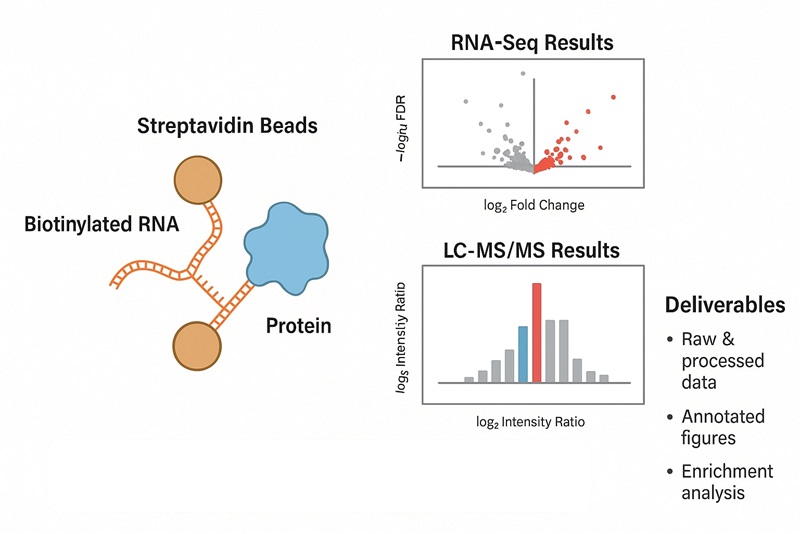
RNA pull-down captures proteins that bind a specific RNA in a biological lysate using a biotinylated RNA bait (in vitro approach) or biotinylated antisense probes that hybridize to the endogenous RNA (capture approach). Bound proteins are eluted and identified by LC–MS/MS, then statistically prioritized against appropriate negative controls.
Use it when you need to:
- Identify RNA-binding proteins (RBPs) for a long noncoding RNA, UTR element, IRES, viral RNA, or structured motif.
- Compare wild-type vs mutated RNA to pinpoint sequence/structure determinants of binding.
- Validate on-target engagement for RNA-targeting molecules (e.g., ASOs, staple constructs) by shifts in the RBP interactome.
- Contextualize hits with functional annotation to nominate regulators and co-factors for downstream validation.
Service Formats: In-Vitro RNA Bait vs Endogenous Capture
In-Vitro Biotinylated RNA Bait Pull-Down
- Custom in-vitro–transcribed or chemically synthesized RNA with 5′/3′ biotin tag.
- Ideal for discrete RNAs (lncRNA domains, UTR fragments, structured elements) and mutant/competitor studies.
- Options: native vs crosslinked lysate input; magnesium/monovalent salt optimization; RNase protection for structured regions.
Endogenous RNA Capture (Probe-Based; CHIRP-style)
- Biotinylated DNA oligo set tiled along the endogenous target RNA to pull down native complexes.
- Optional mild formaldehyde crosslinking to stabilize transient or chromatin-associated interactions.
- Supports follow-on nucleic-acid co-purification (RNA/DNA) if required for orthogonal assays.
Why Choose Creative Proteomics for RNA Pull-Down Studies
- ≤1% Protein-Level FDR: High-confidence identification with stringent false discovery control.
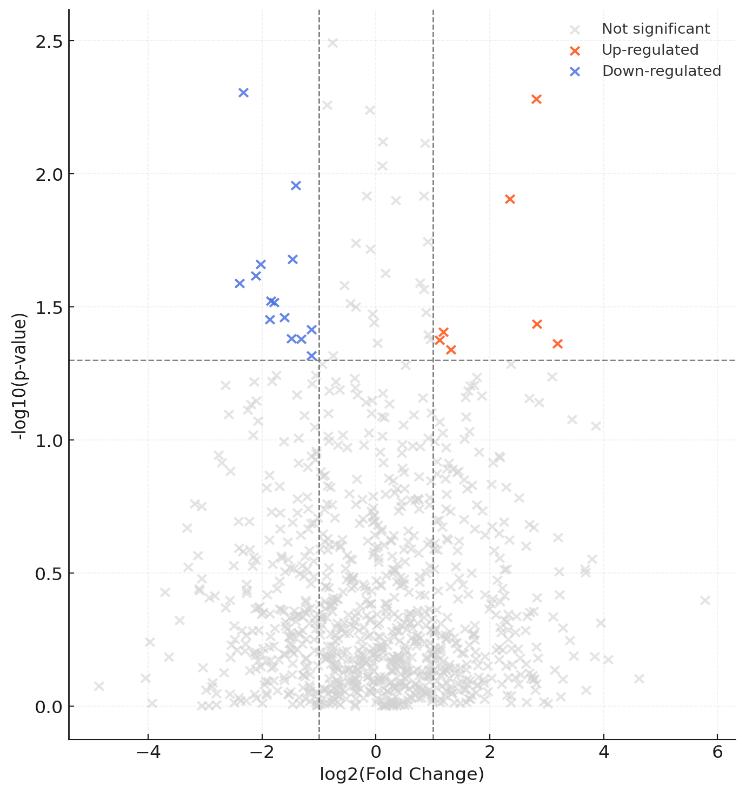
- ≥2× Control-Enriched & Statistically Significant: Final hits require ≥2-fold enrichment vs controls and adjusted q ≤ 0.05.
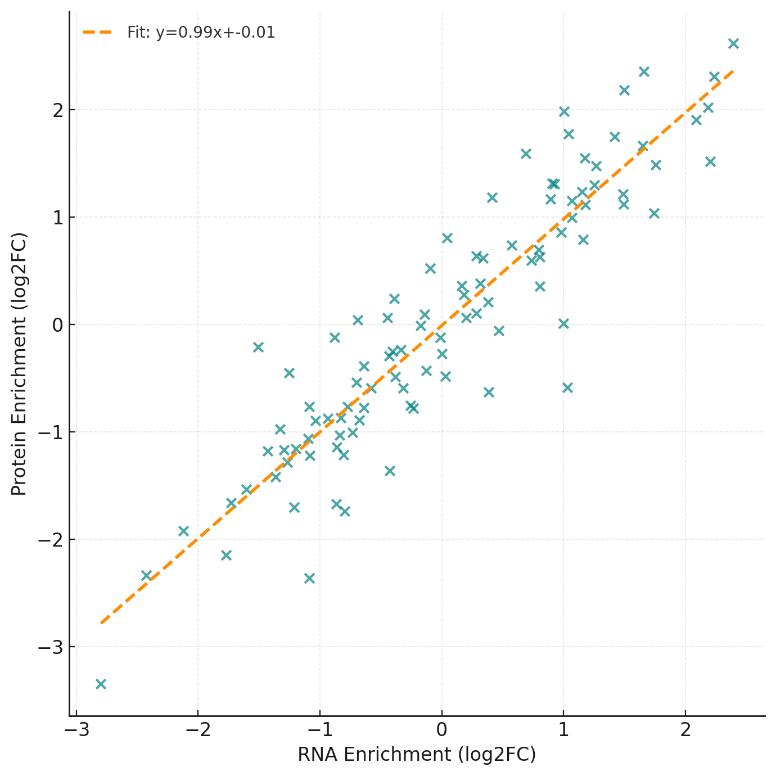
- Replicate Concordance ≥0.85 (Pearson r): Built-in biological reproducibility check for every condition.
- Retention Time Drift ≤ ±90 s: LC–MS system performance continuously monitored for batch stability.
- Quant Precision: CV ≤ 15% (QC Peptides): Instrument variability kept within strict bounds for quantitative reliability.
- Supports 16+ Plex Multiplexing: Enable condition-rich experiments without cross-batch artifacts.
- Validated Wash Resilience: High-confidence binders must persist across ≥2 stringency conditions.
- Off-Target–Screened Probe Designs: All RNA/probe designs undergo hybridization screening prior to use.
- Cold-Competition Confirmable: Optional assays to confirm saturable, specific RNA–protein binding.
- Transparent Contaminant Flagging: No silent filtering—common contaminants are flagged, not hidden.
Custom RNA Bait and Probe Design Options
RNA bait: 80–1,500 nt; 5′/3′ biotinylation; optional stabilizing secondary-structure steps (Mg²⁺ folding, temperature ramps).
Tiled antisense probes (endogenous capture): 20–25-mer; 40–60% GC; tiling density ~1 probe/60–100 nt; off-target screens against host transcriptome.
Mutant/competitor sets: point mutations at motifs (e.g., RBP consensus), truncations to isolate functional domains.
Deliverable: in-silico structure snapshot, off-target report, and final capture bill-of-materials.
Control Strategy and Specificity Validation
Specificity controls: beads-only, scrambled RNA/probes, unrelated RNA, mutated motif.
Affinity validation: cold-competition with unlabeled RNA; concentration series to test saturability.
Context control: capture from perturbed vs baseline lysates to confirm biology-dependent recruitment.
Decision rule: a protein is called a hit if enriched vs ≥2 negative controls (effect-size threshold) under peptide/protein-level FDR and with replicate persistence.
Crosslinking and Stringency Options for Different Affinity Ranges
Native (no crosslink): maximize physiological affinity; choose when expecting strong/stable interactions.
UV 254 nm: map direct contacts; lower background from indirect interactors; mild yield trade-off.
Formaldehyde (reversible): stabilize transient/chromatin-tethered complexes; plan reversal before MS.
Wash ladder: start with physiological salt; escalate ionic strength and mild detergents; lock final stringency where positive controls persist and negatives collapse.
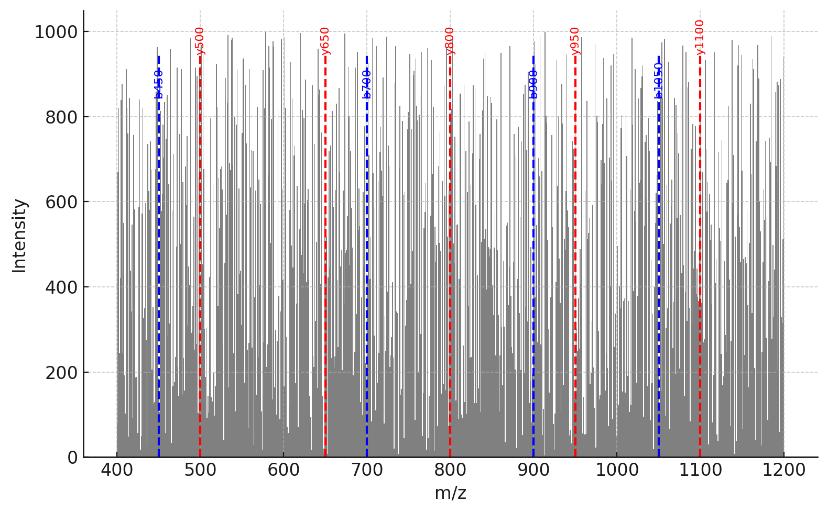
Mass Spectrometry and Quantification Options
Acquisition: HRAM LC–MS/MS; DDA or DIA selectable.
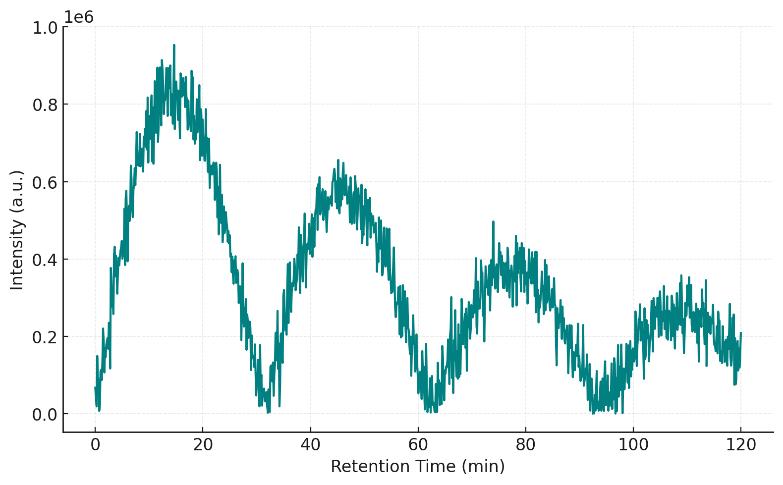
Chromatography: C18 nanoLC, 90–120 min gradients; trap-and-elute to concentrate low-level binders.
Search & FDR: target–decoy; 1% PSM and 1% protein FDR; ≥2 unique peptides preferred for confirmatory calls (documented exceptions).
Quant options: LFQ (intensity-based) or isobaric multiplexing (multi-condition contrasts, batch minimization).
QC Criteria and Acceptance Standards
- LC stability (retention-time drift ≤ ±90 s across batch).
- MS performance (instrument QC peptide CV ≤ 15%).
- Replicate reproducibility (protein-level Pearson r ≥ 0.85 within condition).
- Enrichment robustness (hit persists across ≥2 stringency steps or validated by competition).
- Contaminant filtration against a common contaminant reference (e.g., CRAPome-informed); flagged rather than auto-removed when biology is plausible.
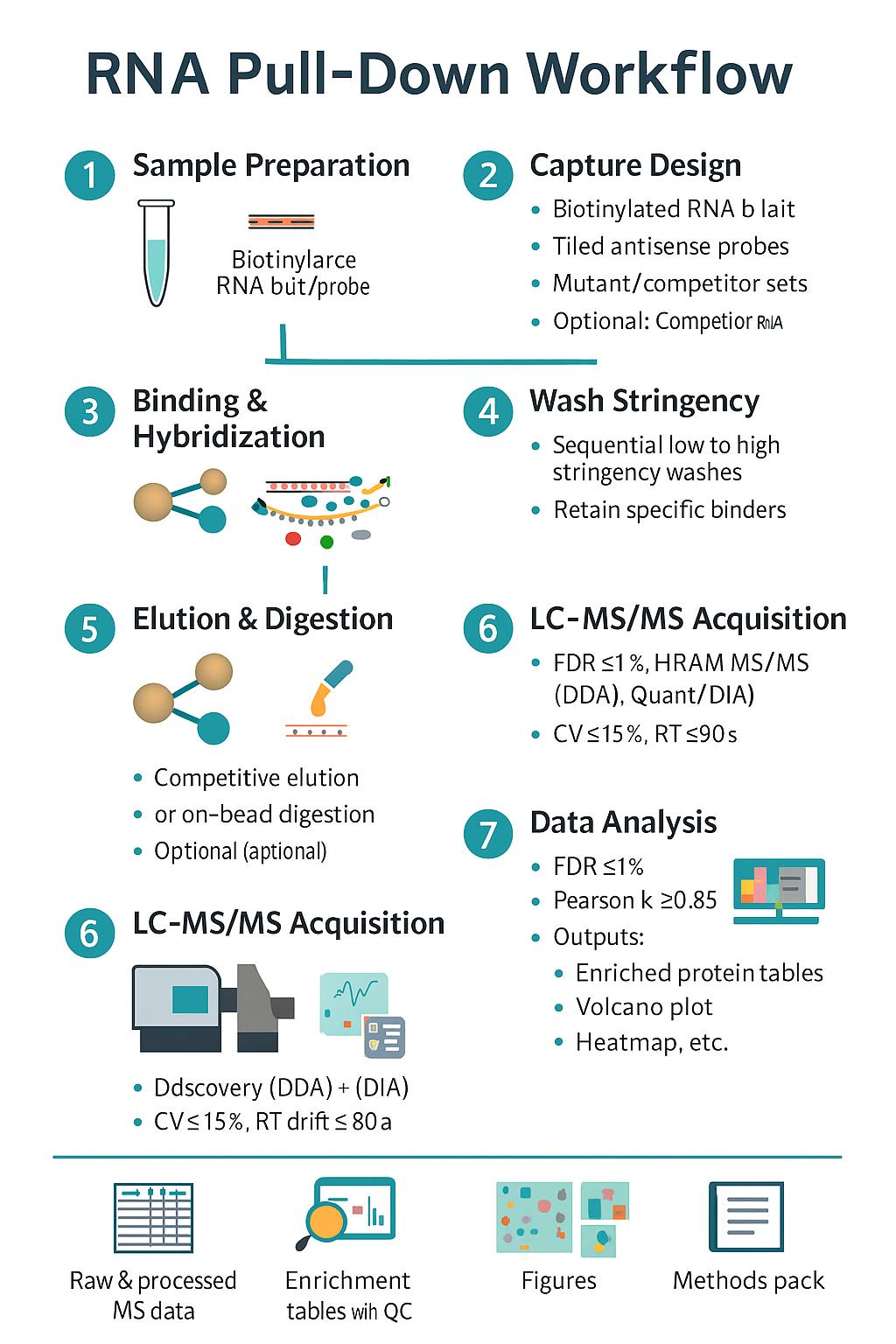
Workflow for RNA Pull-Down Analysis
- Study Definition — biological question, factors, power analysis, covariates, controls.
- Harmonized Sampling — matched aliquots, synchronized processing for RNA and proteins.
- Molecular Extraction — RNA isolation with DNase; protein extraction with denaturation/reduction/alkylation; peptide generation by trypsin/LysC.
- Library/Label Prep — stranded RNA libraries; DIA-ready peptides or TMTpro labeling; optional PTM enrichments.
- Sequencing & LC–MS/MS — platform and gradient matched to depth/throughput goals; routine QC runs.
- Primary Processing — mapping/quantification for RNA; identification/quantification for proteins; strict FDR control.
- Cross-Omics Integration — DEG/DEP calls, concordance/discordance mapping, pathway/network inference, regulator activity scoring.
- Reporting & Handover — raw + processed data, QC dashboards, figures, and an interpretation narrative aligned to decisions you need to make.
Sample Types, Input Amounts, and Pre-Analytical Guidelines
| Category | Details & Recommendations |
|---|---|
| Accepted Sample Types | - Cultured cells (adherent/suspension) - Tissue homogenates - Subcellular fractions (cytosol, nucleus) - Virus-infected cells - Cell-free reconstituted RNPs |
| Minimum Input Amount | - Total protein: ≥1–2 mg per pull-down condition - Volume: 100–500 µL lysate per reaction (concentration-dependent) - Pilot scale available for low-abundance targets |
| Replicates | - ≥3 biological replicates per condition recommended - Technical replicates optional but not a substitute |
| Lysis Requirements | - RNase-free lysis buffers only - Avoid chaotropic agents (e.g., >2 M urea or guanidine) - Glycerol ≤10% (if used) - Include RNase inhibitors (e.g., RNasin) |
| Additives | - Mg²⁺ can be added for preserving RNA structure - DTT/β-mercaptoethanol discouraged (may disrupt interactions) |
| Shipping & Storage | - Snap-freeze lysates immediately after preparation - Ship on dry ice; avoid freeze–thaw cycles - Provide full buffer composition and RNA integrity notes |
| Optional Treatments | - Crosslinking (UV 254 nm or formaldehyde) possible prior to lysis if required - Subcellular fractionation available on request |
Data Analysis and What You Will Receive
Raw Files: vendor MS files + mzML; search outputs.
Processed Tables: protein IDs with gene symbols, peptide counts, abundance metrics, FDR, enrichment vs controls, effect sizes, and adjusted p-values.
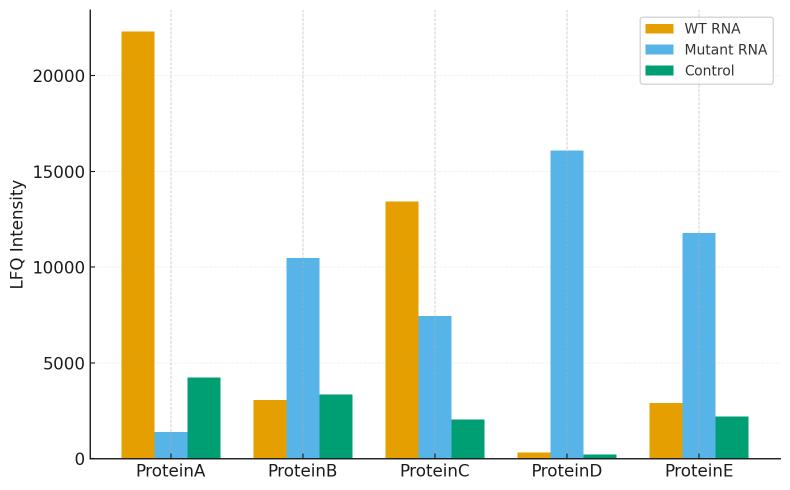
Figures: QC dashboard, replicate correlation, enrichment volcano plots, heatmaps, and interactome/network diagrams.
Annotations: GO terms, complexes, pathways, domains/RNA-binding motifs; contaminant flags.
Methods Package: protocol parameters, capture chemistry, wash ladder, elution mode, instrument settings, and software versions for reproducibility.
Optional Add-Ons: targeted PRM/MRM confirmation, western blot panel, RIP-qPCR for selected RBPs, motif/structure analysis of the RNA region.
Optional Validation and Follow-Up Studies
- Targeted Verification: PRM/MRM assays or immunoblotting on selected interactors.
- Orthogonal Confirmation: RIP-qPCR or RNA footprint assays for RNA-binding validation.
- Functional Linking: Perturbation experiments (e.g., knockdown or chemical treatment) followed by interactome comparison.
- Pathway Integration: Map validated RBPs into pathways to prioritize mechanistic nodes.
Recommended Experimental Designs for Different Study Goals
Motif-Specificity Study: Wild-type RNA vs. motif-mutant ± competitor RNA, ≥3 replicates; multiplexed quantification preferred.
Contextual Interactome Mapping: Endogenous capture ± crosslinking under baseline vs. treatment conditions to reveal context-dependent binders.
Comparative Mechanistic Study: Two or more RNA constructs (domain truncations or isoforms) assayed in parallel, contrasted under identical conditions.
Exploratory Discovery: Broad lysate-based pull-down with stringent controls, followed by DIA quantification for consistent coverage.
Use Cases: When RNA Pull-Down Delivers Maximum Value
lncRNA mechanism mapping
Define effector proteins for an lncRNA domain and nominate perturbation targets.
UTR/IRES biology
Identify translational regulators bound to 5′/3′ UTR elements or IRES segments under defined conditions.
Viral RNA host-factor discovery
Enrich host RBPs that engage structured viral RNAs to inform genetic or chemical screens.
ASO/staple validation
Demonstrate on-target interactome shifts versus scrambled control.
Comparative interactomics
Contrast wild-type and motif-mutant RNAs to pinpoint binding determinants.
Common Pitfalls and Mitigation Strategies
High Background Binding
Mitigation: Increase detergent/salt concentration, use NeutrAvidin beads, include competitor nucleic acids.
Loss of Weak/Transient Interactions
Mitigation: Apply UV crosslinking or on-bead digestion; reduce wash harshness.
Probe Off-Target Capture
Mitigation: Re-design probe sets with adjusted GC content; validate off-targets via qPCR.
Batch Variability in Quantification
Mitigation: Use isobaric multiplexing or robust normalization; include internal QC peptides.
Over-interpretation of Contaminants
Mitigation: Apply CRAPome-informed filtering; report flagged proteins transparently with annotation context.
FAQ of RNA Pull Down Analysis
How do you distinguish true RNA-binding proteins from background?
By combining a control stack (beads-only, scrambled/non-target, mutant/competitor) with quantitative thresholds under FDR control and persistence across replicate/stringency settings. Candidate lists are background-subtracted and statistically ranked.
Can structured or long RNAs be used as bait?
Yes. We evaluate predicted secondary structure, place biotin to minimize steric hindrance, and can include stabilizing cations or folding steps. For very long RNAs, domain-wise tiling improves capture and interpretability.
When is endogenous capture preferred over in-vitro bait?
Choose probe-based capture when you need native RNP context (including co-factors that assemble co-transcriptionally or in specific compartments) or when the RNA is hard to produce in vitro.
What quantification strategy should I pick?
Label-free is efficient for focused designs; isobaric multiplexing is recommended for multi-condition comparisons (e.g., wild-type vs mutant vs competitor) to control batch effects and maximize sensitivity to fold-changes.
How do you handle weak/transient interactions?
Options include UV or reversible crosslinking, reduced washing harshness, on-bead digestion, and lower-temperature handling. We balance these against specificity using orthogonal controls.
Can the service support pathway or complex-level interpretation?
Yes. We annotate enriched complexes and pathways, overlay domain features (e.g., RRM, KH, DEAD-box), and provide network-level views to prioritize mechanistic nodes for follow-up.
Learn about other Q&A about proteomics technology.
Publications
Here are some publications in Proteomics research from our clients:

- In Vitro Identification of Phosphorylation Sites on TcPolβ by Protein Kinases TcCK1, TcCK2, TcAUK1, and TcPKC1 and Effect of Phorbol Ester on Activation by TcPKC of TcPolβ in Trypanosoma cruzi Epimastigotes. 2024. https://doi.org/10.3390/microorganisms12050907
- Metasecretome and biochemical analysis of consortium PM-06 during the degradation of nixtamalized maize pericarp. 2023. https://doi.org/10.1016/j.bcab.2023.102634
- Quantitative Proteomic Analysis of Cellular Responses to a Designed Amino Acid Feed in a Monoclonal Antibody Producing Chinese Hamster Ovary Cell Line. 2018. https://doi.org/10.29252%2F.22.6.385
- Identification of a novel anti-HIV-1 protein from Momordica balsamina leaf extract. 2022. https://doi.org/10.3390/ijerph192215227
- Regulation of cardiac ferroptosis in diabetic human heart failure: uncovering molecular pathways and key targets. 2024. https://doi.org/10.1038/s41420-024-02044-w