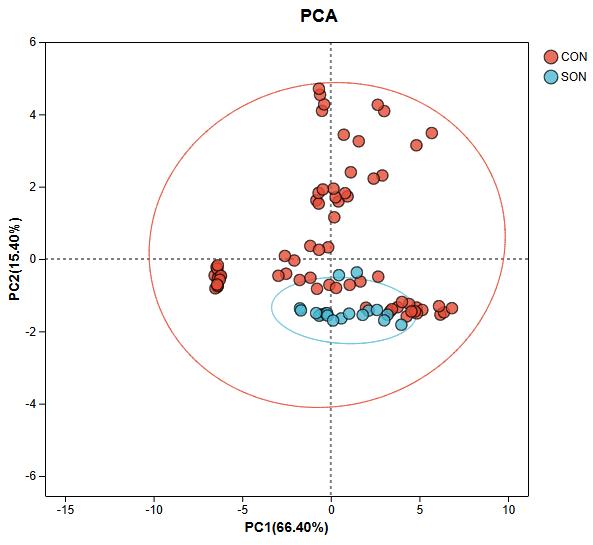
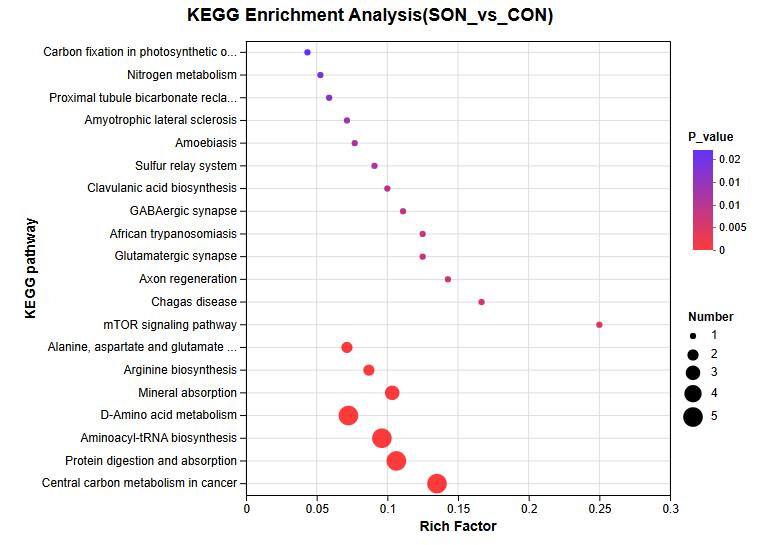
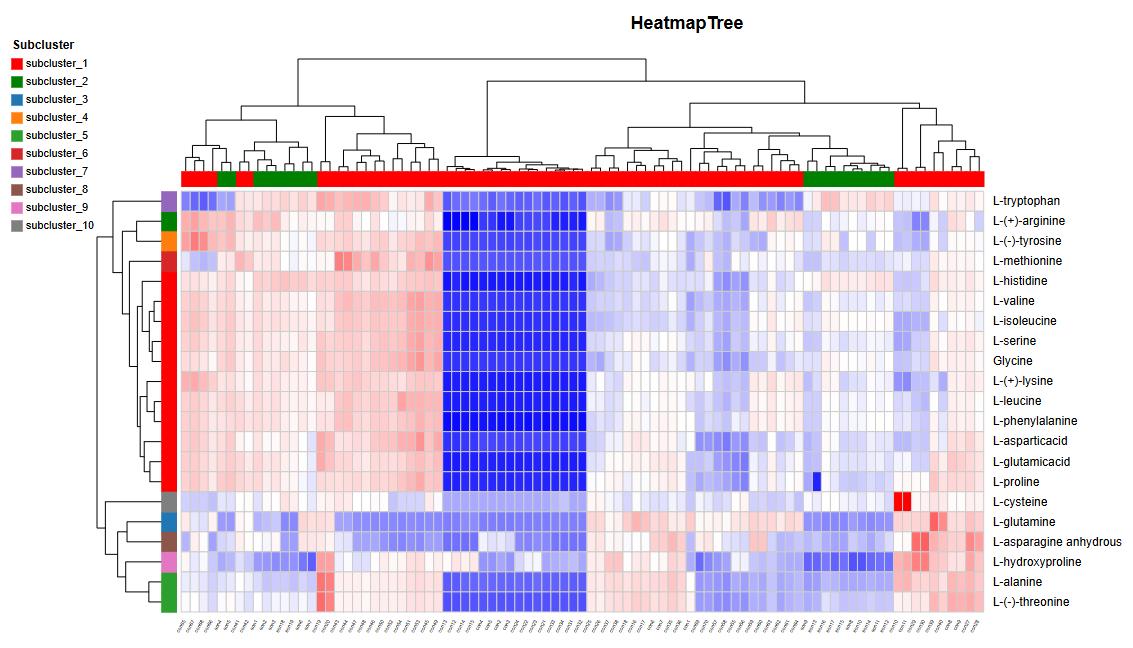
Amino Acid Analysis (AAA) Service
At Creative Proteomics, we've spent over a decade refining amino acid analysis (AAA) to support cutting-edge research and product development across biopharma, clinical research, and environmental sciences. Our comprehensive AAA solutions are designed to deliver reliable, high-throughput data—whether you're optimising monoclonal antibody production or tracking metabolic markers in clinical studies.
By combining powerful technologies like iTRAQ, SRM/MRM, and LC-MS/MS with a broad range of separation and detection methods, we help clients extract detailed insights from even the most complex samples.
Submit Your Request Now
×- Why Analyze
- How to Analyze
- Advantage
- Sample Requirement
- FAQ
- Demo
- Case Study
- Publication
What is Amino Acid Analysis (AAA)?
Amino acids are the essential building blocks of life—literally. These small molecules join together through peptide bonds to form proteins and polypeptides, which drive nearly every function in living organisms. But their role doesn't stop at protein synthesis.
AAs also play a central role in metabolism, serving as the foundation for critical compounds like fatty acids and ketone bodies. Whether freely circulating in biological fluids or present as protein breakdown products, AAs are vital for maintaining internal balance—also known as homeostasis.
Why Analyze Amino Acids?
Amino Acids in Biological and Metabolic Research
In both clinical research, amino acid profiling offers deep insight into human health. Shifts in AA levels have been directly linked to conditions like:
- Phenylketonuria (PKU) – caused by the accumulation of specific AAs
- Type 2 diabetes – where branched-chain AAs often show abnormal levels
- Liver and kidney disorders – which alter AA metabolism
- Cancers, including adenoma and colorectal cancer – where AA uptake changes can signal tumour progression
Importantly, it's not just the standard "essential" or "protein-building" AAs that matter. Researchers are increasingly focused on non-proteinogenic or "unusual" amino acids—byproducts of microbial activity or metabolic disorders—which may hold the key to novel biomarkers or therapeutic targets.
Why It Matters to Drug Developers
For drug developers, especially those optimising cell culture conditions or fine-tuning protein engineering, efficient AAA translates to:
- Faster formulation development
- Higher product consistency
- Better understanding of metabolic pathways
Whether you're refining monoclonal antibody production tips or developing novel peptide drugs, AAA is now a must-have analytical tool.
Industrial Applications of AAs
AAs are more than a topic for the lab—they're big players in biopharma, clinical research, nutrition, and even skincare. In a recent analysis of formulation trends across therapeutic proteins, amino acid manipulation was cited as a key factor in improving cellular health metrics during production. From monoclonal antibody production tips to media optimisation in CHO cell lines, AA profiling is becoming standard practice.
Why Quantifying AAs Is a Game Changer
Whether you're producing biologics or researching metabolic diseases, accurate measurement of AAs in biological samples is foundational. High-throughput, precise technologies are now enabling scientists to:
- Predict and monitor disease progression
- Optimise fermentation or cell culture systems
- Decode metabolic pathways for new drug targets
Understanding amino acids isn't just academic—it's a practical necessity in life sciences R&D. For biopharma teams seeking faster development timelines or deeper biological insights, AA analytics can provide the edge.
How is Our Amino Acid Analysis Performed?
Thanks to recent bioanalytical advances, AAA has become a routine tool across multiple industries—from monoclonal antibody production to clinical research.
Many partners reported integrating mass spectrometry-based AAA into their workflows to improve speed and sensitivity. This shift reflects the broader trend toward high-throughput, low-sample-volume analysis that delivers actionable results fast.
Core Workflow: From Protein to Data
Modern AAA techniques typically follow a four-step process:
Protein or peptide hydrolysis: Breaking down complex molecules into individual amino acids using acid treatment.
- Separation: Using chromatography to isolate each amino acid.
- Derivatization: Chemically tagging AAs to improve visibility (optional—some methods skip this).
- Detection: Measuring concentrations via spectrophotometry or mass spectrometry.
- Mass spectrometry (MS)-based assays are rapidly gaining popularity. These derivatization-free workflows allow for:
- Greater sensitivity
- Lower sample volume requirements
- Direct quantification of individual AA residues using internal standards
 Figure 1. Organigram summarizing the main analytical methods for AAA [1].
Figure 1. Organigram summarizing the main analytical methods for AAA [1].
Sample Types and Flexibility
Today's AAA platforms can handle an impressive range of biological samples:
- Blood components: Serum, plasma, and dry blood spots
- Other fluids: Cerebrospinal fluid and urine
- Tissue extracts: For research purposes
Importantly, these methods can also distinguish between D- and L- amino acid isomers—an essential capability in neurological and microbial studies.
Technologies That Drive Results
Our AAA platform leverages multiple separation strategies, including:
Ion-exchange chromatography
Reverse-phase HPLC
Gas chromatography (GC)
Capillary electrophoresis (CE)
Strong cation exchange (SCX)
Hydrophilic interaction chromatography (HILIC)
For detection and quantification, we integrate:
UV and fluorescence spectrophotometry
Electrospray ionization tandem mass spectrometry (ESI-MS/MS)
MALDI-TOF and TOF/TOF MS/MS
Whether your project calls for ultra-sensitive peptide profiling or routine AA quantification, our automated amino acid analyzers and high-throughput LC-MS/MS systems ensure both accuracy and efficiency.
Top Applications of AAA Services Across Industries
Food Industry: Ensures accurate labeling and nutritional quality.
Pharmaceuticals: Supports drug development by verifying amino acid content.
Clinical Research: Aids in studying metabolism and disorders.
Animal Feed Production: Optimizes feed formulas based on amino acid levels.
Why Choose Creative Proteomics for AAA Services?
- End-to-end support: From sample prep to data interpretation
- Flexible sample types: No need to conform to a rigid protocol
- Regulatory-grade accuracy: Trusted by CROs, biotech firms, and academic labs worldwide
- Professional detection and analysis capability: Experienced technical team, strict quality control system, together with ultra-high resolution detection system and professional data pre-processing and analysis capability, ensure reliable and accurate data.
- Reproducible: By adding isotope internal standard for correction, various samples can be accurately quantified.
- High-throughput: Deeper coverage for protein or peptide sequence.
- High resolution and sensitivity: Over 100 types of amino acids and derivatives can be quantitatively analyzed at the same time.
Sample Requirements for Amino Acid Analysis
| Sample Type | Sample Format | Sample Amount | Preparation Requirements | Notes |
|---|---|---|---|---|
| Dry Protein/Peptide Samples | Solid | ≥100 µg at 0.5-2 mg/mL | Ensure high purity; lyophilized and free of contaminants | Store dry; avoid moisture exposure |
| Liquid Protein/Peptide Samples | Solution | >100 µL at minimum 0.5 mg/mL | Deionized water or suitable buffer; avoid interfering agents | Fresh or properly preserved |
| Biological Fluids (e.g., plasma, urine) | Solution | 50-200 µL | Centrifuged to remove particulate matter | Use fresh or flash-frozen samples |
| Tissue Samples | Solid or Solution | 10-50 mg | Homogenized, lyophilized, or flash-frozen | Avoid repeated freeze-thaw cycles |
| Food Samples | Solid | 100-200 mg | Dried and ground into fine powder | Avoid additives or preservatives |
| Environmental Samples (e.g., soil, water) | Varies | Varies based on sample type | Pre-treated to remove any interfering substances | Filtered and clarified if in solution |
How to place an order
The provision of comprehensive support tailored to your specific requirements for Amino Acid Analysis is our area of expertise. Please feel free to contact us via email whenever you need to discuss your specific requirements. Our customer service representatives are available 24 hours a day, from Monday to Sunday.

FAQ on Amino Acid Analysis
What sample types are suitable for amino acid analysis?
Amino acid analysis can be conducted on various sample types, such as:
- Purified proteins
- Peptides
- Biological fluids (plasma, urine)
- Food products
- Environmental samples
It's crucial to ensure that samples are free from contaminants. Preparation steps like homogenizing tissue samples or drying food samples are key to ensuring accurate results.
How does acid hydrolysis affect amino acid analysis results?
Acid hydrolysis is a common method used to break down proteins and peptides into individual amino acids. While effective for most amino acids, it can cause degradation of sensitive ones, such as tryptophan and cysteine. These amino acids are particularly vulnerable to acidic conditions. To preserve them for accurate quantification, alternative hydrolysis methods or derivatization techniques can be used.
How do sample preparation techniques affect amino acid analysis results?
Sample preparation significantly impacts the accuracy and reliability of amino acid analysis. For protein samples, acid hydrolysis is often used, but this process can degrade sensitive amino acids. Optimizing hydrolysis conditions, such as temperature and acid type, is essential. Moreover, contaminants like lipids or carbohydrates can interfere with chromatographic analysis, so purification methods such as centrifugation and filtration are critical before analysis.
Can amino acid analysis detect non-proteinogenic amino acids? If so, how?
Yes, amino acid analysis can detect non-proteinogenic amino acids, which often arise from metabolic processes or microbial metabolism. Advanced techniques like pre-column derivatization and mass spectrometry enhance the detection of these non-standard amino acids. Derivatization improves separation and sensitivity, and using agents like 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) ensures a broader detection range, including less detectable amino acids.
What techniques are used to differentiate between D- and L-amino acids in samples?
Differentiating D- and L-amino acids typically involves chiral chromatography or mass spectrometry. Chiral chromatography separates these forms based on their stereochemistry, while mass spectrometry identifies them by their unique mass-to-charge ratios. This differentiation is crucial for studying metabolic pathways and understanding biochemical processes.
How do you ensure the accuracy and reproducibility of amino acid quantification?
To ensure accuracy and reproducibility, we implement rigorous quality control measures:
Internal standards (isotopically labeled amino acids) help account for any sample preparation or analysis variability.
Calibration curves are used to maintain precision.
Multiple measurement replicates confirm reproducibility.
Our expert team continuously reviews protocols to maintain high data quality standards.
Learn about other Q&A about proteomics technology.
Demo
Case Study

Amino acid analysis for peptide quantitation using reversed-phase liquid chromatography combined with multiple reaction monitoring mass spectrometry
Journal: Analytical and Bioanalytical Chemistry
Published: 2023
DOI: 10.1007/s00216-023-04840-2
- Abstract
- Methodology
- Conclusion
Amino acid analysis (AAA) is critical for peptide quantification in research and drug development. However, traditional methods often require complex derivatization procedures, which can introduce instability, low reproducibility, and lengthy preparation times. In this case, a team of researchers sought to develop a simplified method to quantify amino acids, including sulfur-containing amino acids (methionine and cysteine), from peptide hydrolysates without the need for derivatization. This would offer a faster, more robust solution for high-throughput analysis.
Peptide Hydrolysis: Peptides were hydrolyzed using 6 M hydrochloric acid to release amino acids.
Internal Standards: Isotopically labeled amino acids (^13C, ^15N) were spiked into the samples to ensure accurate quantification.
Chromatographic Separation: Reversed-phase ultra-performance liquid chromatography (RP-UPLC) was employed using a C18 column.
Detection and Quantification: MRM-MS was used for amino acid detection, with quantification based on peak area ratios of analyte to internal standards.
The developed RP-UPLC-MRM-MS method provided a derivatization-free and high-throughput solution for the absolute quantification of amino acids from peptide hydrolysates. This approach successfully quantified 17 amino acids, including methionine and cysteine in their oxidized forms, without the need for external calibration. The method was robust, reproducible, and simple to implement, making it ideal for routine peptide quantification in both research and pharmaceutical applications.
 Figure 1. The Graphical Abstract of Amino Acid Analysis.
Figure 1. The Graphical Abstract of Amino Acid Analysis.
Publication
See more articles published by our clients.

- KURIL, Akhilesh Kumar, and K. SARAVANAN. High-throughput method for Peptide mapping and Amino acid sequencing for Calcitonin Salmon in Calcitonin Salmon injection using Ultra High Performance Liquid Chromatography–High Resolution Mass Spectrometry (UHPLC-HRMS) with the application of Bioinformatic tools. Journal of Pharmaceutical and Biomedical Analysis. 2024. DOI: https://doi.org/10.1016/j.jpba.2024.116094
- MALDONADO, Edio, et al. Trypanosoma cruzi DNA Polymerase β Is Phosphorylated In Vivo and In Vitro by Protein Kinase C (PKC) and Casein Kinase 2 (CK2). Cells. 2022. OI: https://doi.org/10.3390/cells11223693
- YANG, Zhi, et al. Reducing branched-chain amino acids improves cardiac stress response in mice by decreasing histone H3K23 propionylation. The Journal of Clinical Investigation. 2023. DOI: https://www.jci.org/articles/view/169399
- HERRERA, Victoria LM, et al. Confirmation of translatability and functionality certifies the dual endothelin1/VEGFsp receptor (DEspR) protein. BMC Molecular Biology. 2016. DOI: https://doi.org/10.1186/s12867-016-0066-8