What is Capsanthin?
Capsanthin is a natural red pigment found in chili peppers, which is the material basis for the red color of chili peppers' fruits, and is crucial to the appearance and quality of chili peppers' fruits, and plays a vital role in protecting chili peppers from environmental stresses and regulating the ripening process of the fruits. It is widely used in food processing, cosmetic and pharmaceutical industries because of its strong coloring ability and safety for human beings
Analysis of capsanthin is critical to understanding the physiology, biochemistry, and impact on human health of the pepper plant. Analyzing capsanthin provides you with valuable information on how the pepper plant adapts to different environmental conditions (e.g., light, temperature, and nutrient availability) and how it regulates its growth, development, and fruit ripening. In addition, it provides insights into the metabolic pathways involved in the body's response to oxidative stress and inflammation.
By analyzing and quantifying capsanthin and their metabolites and determining their patterns of change under specific chili pepper growth conditions, environmental factors, or human health conditions, you can lay the scientific groundwork for improving chili pepper plant resistance, increasing the nutritional and commercial value of chili peppers, and promoting human health.
Why Choose Us?
- High Sensitivity: Detection limits as low as 0.1 ng/mL using advanced LC-MS/MS systems.
- Comprehensive Coverage: Simultaneous analysis of capsanthin isomers, esters, and degradation products.
- Rapid Turnaround: Optimized workflows ensure results within 10–20 business days.
- Multi-Method Validation: Cross-validated results from HPLC, NMR, and MS platforms for unmatched accuracy.
- Custom Solutions: Tailored protocols for complex matrices (e.g., oils, plant extracts, processed foods).
Capsanthin Analysis Services by Creative Proteomics
- Quantitative Analysis of Capsanthin: Accurate determination of capsanthin concentration in different matrices.
- Capsanthin Purity Testing: Identification of purity levels in food additives and nutraceuticals.
- Metabolite Profiling: Comprehensive analysis of capsanthin-related metabolic pathways.
- Structural Characterization: Advanced spectroscopic analysis for molecular structure elucidation.
- Capsanthin Stability Studies: Investigation of capsanthin stability under different environmental conditions.
Detectable Capsanthin, Related Metabolites, and Metabolic Pathways
| Compound Name |
Metabolic Pathway |
Function/Significance |
| Capsanthin |
Carotenoid Biosynthesis |
Primary red pigment in red peppers, antioxidant properties |
| Capsorubin |
Carotenoid Biosynthesis |
Red xanthophyll, contributes to pepper coloration |
| β-Cryptoxanthin |
Carotenoid Metabolism |
Precursor to vitamin A, antioxidant |
| Zeaxanthin |
Xanthophyll Cycle |
Enhances eye health, protects against oxidative stress |
| Violaxanthin |
Xanthophyll Cycle |
Involved in photoprotection in plants |
| Lutein |
Xanthophyll Cycle |
Antioxidant, important for vision and skin health |
| Neoxanthin |
Carotenoid Biosynthesis |
Intermediate in the biosynthesis of ABA (abscisic acid) |
| Antheraxanthin |
Xanthophyll Cycle |
Involved in non-photochemical quenching in plants |
| Phytoene |
Early Carotenoid Biosynthesis |
Precursor to all carotenoids |
| Phytofluene |
Early Carotenoid Biosynthesis |
Intermediate in lycopene biosynthesis |
| Lycopene |
Carotenoid Biosynthesis |
Precursor for β-carotene, strong antioxidant |
| β-Carotene |
Carotenoid Metabolism |
Precursor to vitamin A, essential for human nutrition |
| α-Carotene |
Carotenoid Metabolism |
Pro-vitamin A carotenoid, antioxidant |
| Abscisic Acid (ABA) |
Carotenoid Degradation Pathway |
Plant hormone derived from carotenoids, regulates stress responses |
Methods Used for Capsanthin Analysis
Ultra-Performance Liquid Chromatography (UPLC-QTOF-MS) – High-resolution separation and mass spectrometry-based identification.
High-Performance Liquid Chromatography (HPLC-DAD) – UV-visible detection for precise quantification.
Gas Chromatography-Mass Spectrometry (GC-MS) – For volatile carotenoid derivatives.
 1260 Infinity II LC System (Figure from Agilent)
1260 Infinity II LC System (Figure from Agilent)
 Waters Xevo G2-XS QTOF (Figure from Waters)
Waters Xevo G2-XS QTOF (Figure from Waters)
Workflow for Capsanthin Analysis Service
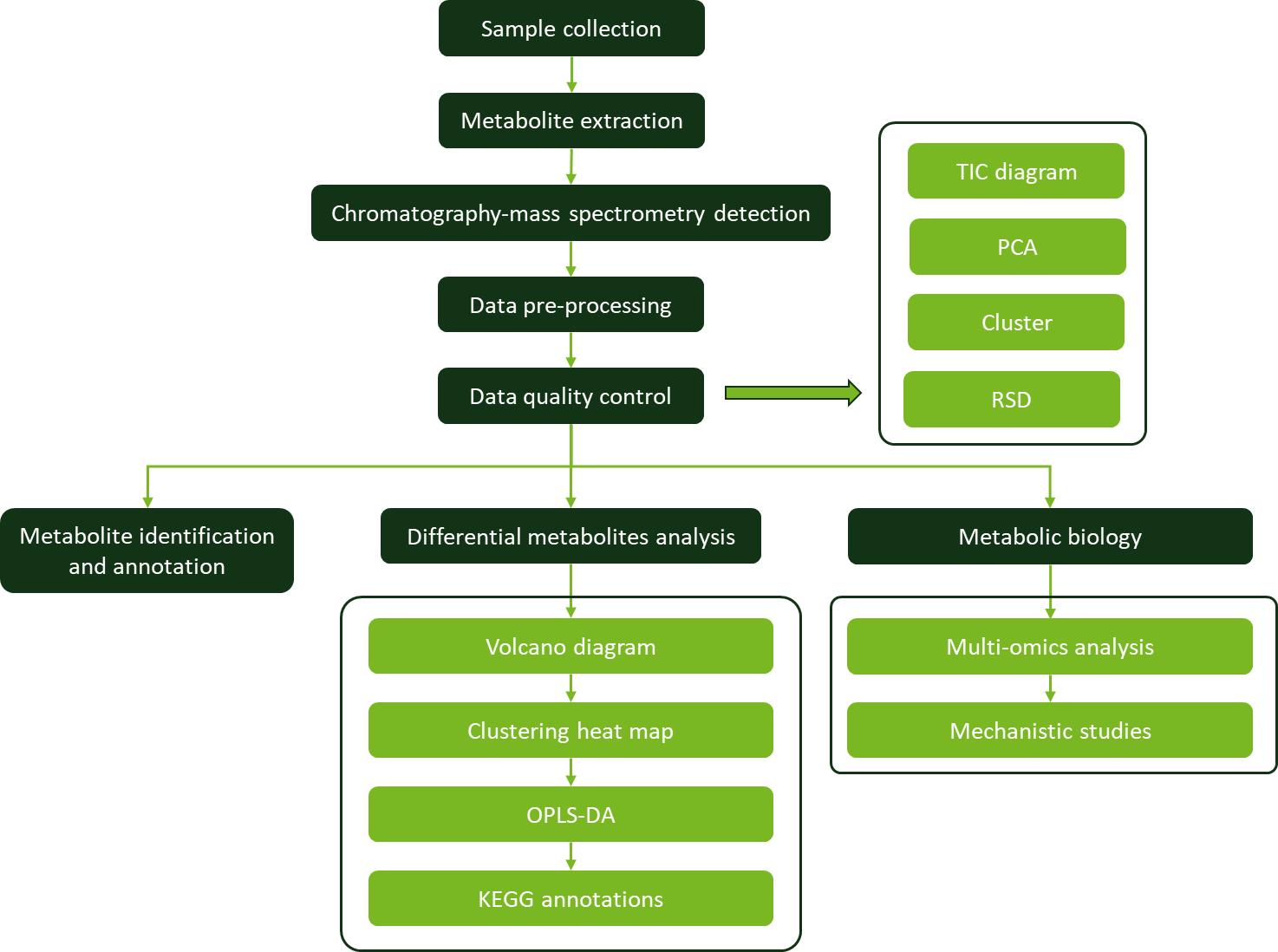
Sample Requirements for Capsanthin Assay
| Sample Type |
Required Amount |
Storage Conditions |
Notes |
| Plant Tissues (e.g., Red Pepper, Chili, Paprika) |
≥ 100 mg (dry) or ≥ 1 g (fresh) |
-80°C (long-term) or -20°C (short-term) |
Freeze-dried samples preferred to prevent degradation |
| Food Products (e.g., Sauces, Spices, Beverages) |
≥ 5 g |
4°C or -20°C |
Avoid light exposure to prevent oxidation |
| Nutraceuticals (Capsules, Tablets, Powders) |
≥ 10 capsules/tablets or ≥ 500 mg powder |
Room temperature (sealed) |
Provide ingredient list if available |
| Liquid Samples (Juices, Extracts, Fermentation Broths) |
≥ 10 mL |
4°C or -20°C |
Store in amber vials to minimize light degradation |
| Purified Capsanthin Standards |
≥ 1 mg |
-20°C or -80°C |
Dissolved in appropriate solvent (e.g., ethanol) |
Applications of Capsanthin Analysis
Case. Simultaneous extraction of edible oil from avocado and capsanthin from red bell pepper using supercritical carbon dioxide as solvent
Background:
Vegetable extracts containing bioactive compounds and supercritical CO2 were analyzed.
The study aimed to obtain capsanthin-rich avocado oil using scCO₂ extraction and examine the effect of avocado oil as a co-solvent for capsanthin extraction from red bell pepper.
Samples:
Avocado (variety Haas) from Jaguacy Brasil and red bell pepper from the local market in Campinas/SP, Brazil were used. The pulps were freeze-dried after processing.
Technical methods procedure:
The moisture content was determined by gravimetric method for fresh samples.
The freeze-dried samples was determined by Karl Fischer method.
Particle size was measured by ASAE method after crushing/grinding and sieving.
Bed density and porosity were calculated.
Extraction experiments were conducted in a fixed bed extractor at 50 °C and 400 bar with a 1.5 L/min CO₂ flow rate. Extraction curves were constructed by collecting samples at different times and measuring extract and solvent masses.
Lipid content of avocado was determined by Soxhlet method.
Capsanthin content in raw material was measured by exhaustive extraction with acetone followed by isolation, identification and quantification using open-column chromatography and UHPLC-PDA.
Results:
The moisture contents of avocado and red bell pepper decreased after freeze-drying.
Avocado had a high lipid content (68%), and red bell pepper had a certain capsanthin level (625 ± 87 g/g in freeze-dried).
The extraction curves showed the oil solubility in scCO₂ and the extraction kinetics of both materials.
The overall extraction yield of avocado oil was 57% with 84.3% recovery. Capsanthin extraction yield was low (15%) without co-solvent.
In simultaneous extraction, as the proportion of red bell pepper to avocado decreased, capsanthin recovery increased (from 15% to 32%, 35%, and 56% with increasing avocado concentration).
Capsanthin concentration in the extract decreased with more avocado oil as co-solvent due to dilution.
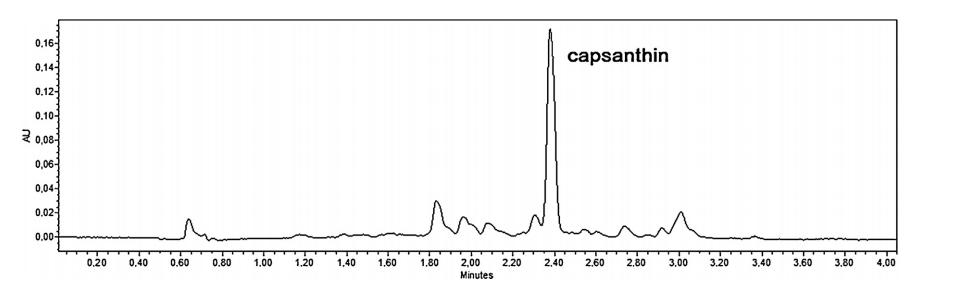 Chromatogram of the freeze-dried red pepper extract.
Chromatogram of the freeze-dried red pepper extract.
Reference
- Barros H D F Q, et al. "Simultaneous extraction of edible oil from avocado and capsanthin from red bell pepper using supercritical carbon dioxide as solvent." Journal of Supercritical Fluids 107 (2016): 315 - 320.


 1260 Infinity II LC System (Figure from Agilent)
1260 Infinity II LC System (Figure from Agilent) Waters Xevo G2-XS QTOF (Figure from Waters)
Waters Xevo G2-XS QTOF (Figure from Waters)

 Chromatogram of the freeze-dried red pepper extract.
Chromatogram of the freeze-dried red pepper extract.