What is Farnesol?
Farnesol is a naturally occurring acyclic sesquiterpene alcohol with a molecular formula of C15H26O. This compound, a colorless liquid under standard conditions, is noted for its hydrophobic nature, rendering it insoluble in water while miscible with various oils. Farnesol is biosynthesized from isoprene units and serves as a critical intermediate in the production of squalene, a precursor to steroids in plants, animals, and fungi. Its diverse applications span across industries, including perfumery, pest control, and potential therapeutic research.
Farnesol analysis is crucial for assessing its purity and concentration in various applications, including perfumery, pest control, and therapeutic research. Accurate analysis ensures quality control, effective use in products, and advances research into its biological functions and potential therapeutic benefits.
Farnesol Analysis Service Offered by Creative Proteomics
Creative Proteomics offers a specialized farnesol analysis service as part of our comprehensive plant target metabolomics platform. This service is meticulously designed to deliver precise and dependable data on the content, composition, and purity of farnesol, catering to the unique requirements of clients across diverse industries.
Categories of Farnesol Analysis Services
Farnesol Qualitative Analysis: Identification of farnesol and its isomers in various samples, including essential oils, fruits, and industrial products.
Farnesol Quantitative Analysis: Measurement of farnesol concentrations in different matrices, providing accurate data for formulation and quality control purposes.
Farnesol Purity Testing: Assessment of farnesol purity to ensure compliance with industry standards and specifications.
Farnesol Isomer Profiling: Detailed analysis of α- and β-farnesol isomers, crucial for applications in fragrance and flavor industries.
Analytical Techniques
Gas Chromatography-Mass Spectrometry (GC-MS):
Purpose: To determine the composition and concentration of farnesol in complex mixtures.
Procedure: Samples are first volatilized and then separated using gas chromatography. The separated compounds are identified and quantified through mass spectrometry.
Instrument Model:
- Agilent 7890A GC/5975C MSD
- Thermo Scientific TSQ 8000 GC-MS
Liquid Chromatography-Mass Spectrometry (LC-MS):
Purpose: LC-MS combines the separation capabilities of liquid chromatography with the detection power of mass spectrometry, offering a highly sensitive and specific analysis of farnesol and its derivatives.
Procedure: In LC-MS, farnesol is first separated in a liquid chromatography system, where it is eluted based on its chemical properties. The separated compound is then ionized and detected using mass spectrometry. This technique provides detailed information on the molecular weight, structure, and concentration of farnesol.
Instrument Model:
- Agilent 6460 Triple Quadrupole LC/MS
- Thermo Scientific Q Exactive Series
Significance of Farnesol Analysis
Perfumery and Fragrance Formulation: Farnesol contributes to the scent profile of floral and citrus fragrances. Accurate analysis ensures the consistency and quality of fragrance products.
Industrial Applications: Farnesol is utilized as a feedstock in the production of specialty chemicals, including cosmetic oils, lubricants, and renewable fuels. Analytical precision is crucial for optimizing industrial processes.
Pest Control: As a natural pesticide, farnesol's effectiveness against pests necessitates accurate quantification for agricultural applications.
Biochemical Research: Understanding farnesol's role in biological processes and its potential therapeutic applications, such as in Parkinson's disease research, requires precise analytical techniques.
Sample Requirements for Farnesol Analysis
| Sample Types |
Volume/Weight Requirements |
Storage Conditions |
Biological Repeat |
| Animal Samples |
-Tissue samples (e.g., liver, adipose tissue) |
1-5 g |
- Store at -80°C or in liquid nitrogen. |
3-6 |
| Plant Samples |
- Leaf, fruit, or flower samples
- Essential oil extracts |
1-5 g (solid) or 1-2 mL (liquid) |
- Store at -20°C for plant material.
- Essential oil extracts can be stored at 4°C. |
| Cell Samples |
- Cultured cells (adherent or suspension)
- Cell lysates |
1-10 million cells or 1-5 mL media |
- Store at -80°C for cell lysates.
- For cultured cells, maintain in appropriate growth conditions until processed. |
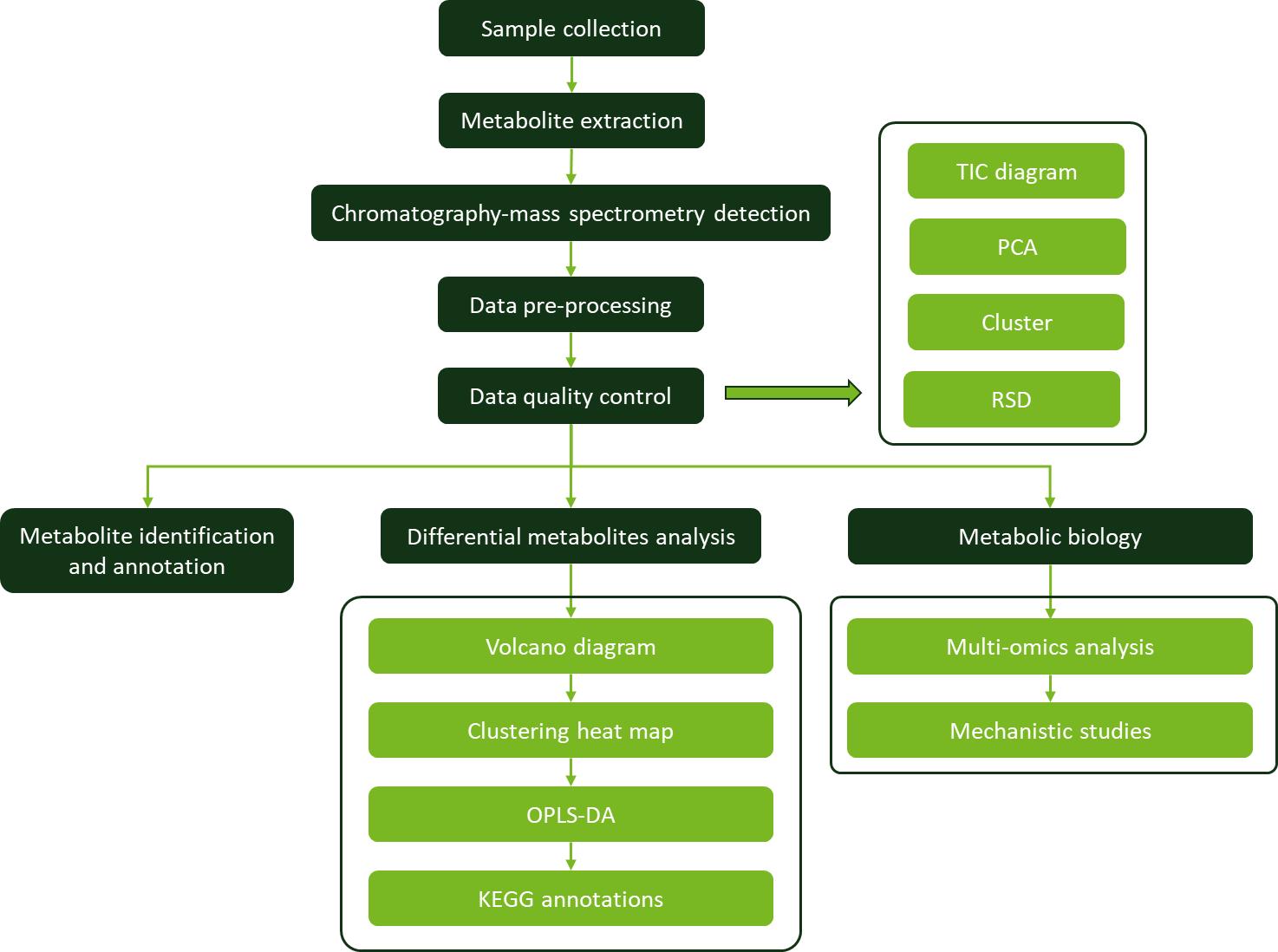
Case. Farnesol is glucuronidated in human liver, kidney and intestine in vitro, and is a novel substrate for UGT2B7 and UGT1A1
Background
Farnesol, an isoprenoid found in plants and produced in humans, has notable anticancer properties and plays a role in cholesterol synthesis. Despite its significance, its metabolism, particularly in humans, has not been thoroughly studied. While previous research identified farnesol metabolism in rabbits, human-specific pathways remained unexplored.
Samples
Human tissue microsomes from liver, kidney, and intestine, as well as cell lines expressing human UGTs.
Technical Methods Procedure
Sample Preparation: Microsomes were prepared from human tissue samples and sonicated. Protein concentrations were determined using standard methods.
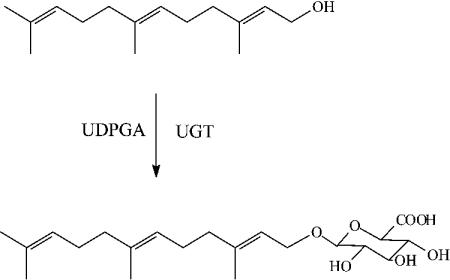
Farnesol Glucuronidation Assays: Farnesol was incubated with microsomes or cell lysates in the presence of UDPGA.
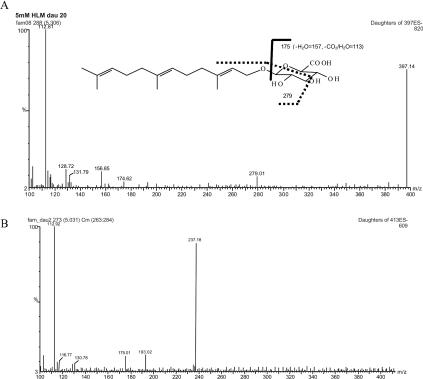
LC–MS/MS Analysis: A sensitive LC–MS/MS method was developed to quantify farnesyl glucuronide with a limit of detection of 30 fmol. The mass spectrum confirmed the structure of farnesyl glucuronide.
Kinetic Studies: Farnesol glucuronidation kinetics were studied using varying concentrations of farnesol to determine Km and Vmax values.
Inhibition Studies: The role of specific UGT isoforms was examined using competitive inhibition assays with known probe substrates. The effects of NADPH on glucuronidation were also tested.
Detection of Novel Products: To identify potential oxidative metabolites, LC-MS was employed to analyze reactions with and without UDPGA.
Results
Glucuronidation Efficiency: UGT1A1 and UGT2B7 were the primary enzymes responsible for farnesol glucuronidation in vitro.
Kinetic Parameters: Farnesol glucuronidation exhibited substrate inhibition in some systems. UGT1A1 primarily contributes to glucuronidation in liver microsomes, while UGT2B7 plays a significant role in the intestine.
Metabolic Products: Farnesol was metabolized to farnesyl glucuronide, hydroxyfarnesol, and hydroxyfarnesyl glucuronide.
Glucuronidation Profile: Farnesyl glucuronide formation rates varied among different UGT isoforms and human tissues, with UGT2B7 showing the highest activity.
Reference
- Staines, Adam G., et al. "Farnesol is glucuronidated in human liver, kidney and intestine in vitro, and is a novel substrate for UGT2B7 and UGT1A1." Biochemical Journal 384.3 (2004): 637-645.







