What is Capsorubin?
Capsorubin is a natural xanthophyll-class carotenoid primarily found in red peppers (Capsicum annuum) and certain lilies. It is a key component of paprika oleoresin, contributing to the vibrant red coloration of mature pepper fruits. Structurally, it belongs to the ketocarotenoid family, with the molecular formula C40H56O4, and exhibits potent antioxidant and anti-inflammatory properties. As a critical metabolite in the carotenoid biosynthesis pathway, capsorubin plays a role in plant stress responses and has applications in food, cosmetics, and agricultural research.
Why Choose Us?
- High Sensitivity and Precision: Using LC-MS/MS and HPLC-DAD, we achieve detection limits as low as 0.1 ng/mL, ensuring accurate detection even at trace levels.
- Comprehensive Metabolite Detection: We identify zeaxanthin's metabolites and precursors, such as lutein and violaxanthin, with 98%+ accuracy, offering a complete metabolic profile.
- High-Throughput Capacity: Our systems process up to 500 samples per day, ensuring fast turnaround without compromising data quality.
- Superior Resolution and Reproducibility: With >10,000 plates in HPLC/UPLC, we provide precise separation and reproducible results across various sample types.
- Quick Turnaround: Results are delivered in 10–14 business days, ideal for fast-paced research or commercial projects.
Capsorubin Analysis Services by Creative Proteomics
- Quantitative Analysis of Capsorubin – Accurate measurement of capsorubin content in different matrices.
- Identification of Capsorubin Derivatives – Detection and structural elucidation of related metabolites.
- Capsorubin Stability Testing – Analysis under different environmental conditions (e.g., light, temperature, pH).
- Capsorubin Extraction Efficiency Assessment – Evaluation of different extraction techniques for maximum yield
- Metabolic Pathway Analysis – Profiling of capsorubin-associated metabolites in biological samples.
- Capsorubin Bioavailability Studies – Examination of absorption, distribution, and metabolism in experimental models.
List of Detected Capsorubin and Related Metabolites
| Compound Name |
Category |
Biological Relevance |
| Capsorubin |
Xanthophyll Carotenoid |
Major red pigment in peppers |
| Capsanthin |
Xanthophyll Carotenoid |
Co-existing carotenoid with capsorubin |
| Violaxanthin |
Xanthophyll Carotenoid |
Precursor in carotenoid biosynthesis |
| Antheraxanthin |
Xanthophyll Carotenoid |
Intermediate in the xanthophyll cycle |
| Zeaxanthin |
Xanthophyll Carotenoid |
Important in plant photoprotection |
| β-Cryptoxanthin |
Xanthophyll Carotenoid |
Pro-vitamin A precursor |
| Lutein |
Xanthophyll Carotenoid |
Plays a role in plant defense |
| β-Carotene |
Carotenoid |
Precursor to vitamin A |
Techniques and Instrumentation for Capsorubin Analysis
Ultra-Performance Liquid Chromatography (UPLC-QTOF-MS) – High-resolution separation and detection of capsorubin and metabolites.
High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) – Accurate quantification based on UV-Vis absorption properties.
Gas Chromatography-Mass Spectrometry (GC-MS) – Complementary technique for volatile metabolite profiling.
 Agilent 1260 Infinity II HPLC (Figure from Agilent)
Agilent 1260 Infinity II HPLC (Figure from Agilent)
 Waters ACQUITY UPLC System (Figure from Waters)
Waters ACQUITY UPLC System (Figure from Waters)
 Agilent 7890B-5977B (Figure from Agilent)
Agilent 7890B-5977B (Figure from Agilent)
 SCIEX Triple Quad™ 6500+ (Figure from Sciex)
SCIEX Triple Quad™ 6500+ (Figure from Sciex)
Workflow for Capsorubin Analysis Service
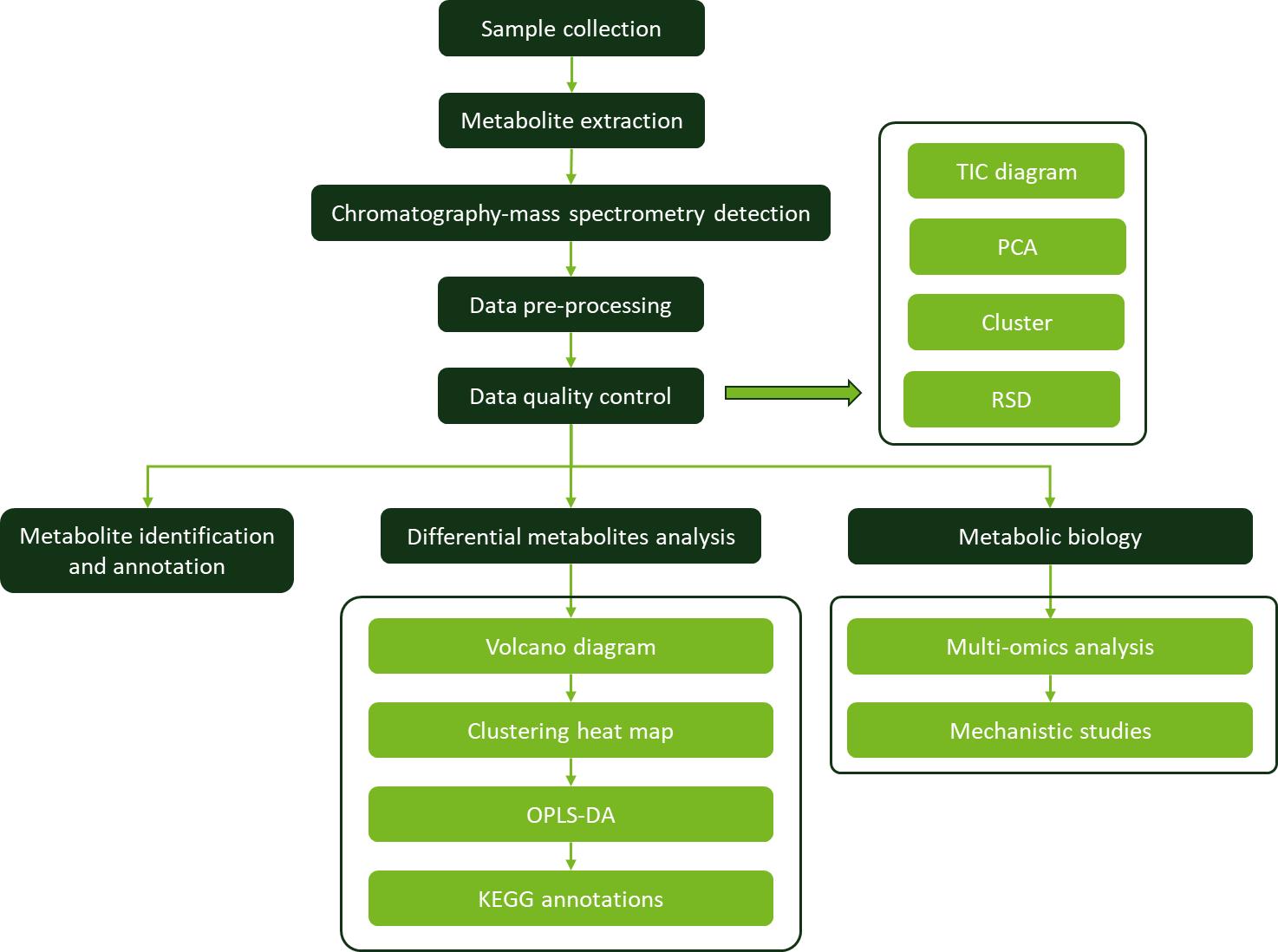
Sample Requirements for Capsorubin Assay
| Sample Type |
Minimum Required Amount |
Recommended Storage Conditions |
Shipping Conditions |
| Plant Tissues (Fresh/Frozen) |
500 mg |
-80°C (Frozen) |
Dry Ice |
| Dried Plant Materials |
100 mg |
Room Temperature |
Sealed, Cool, and Dry |
| Food Samples (e.g., Pepper Extracts, Beverages) |
5 g |
-20°C (Frozen) |
Ice Pack or Dry Ice |
| Dietary Supplements (Powder/Tablets/Capsules) |
1 g or 10 capsules |
Room Temperature |
Sealed, Cool, and Dry |
| Biological Fluids (e.g., Serum, Plasma) |
200 µL |
-80°C (Frozen) |
Dry Ice |
| Cell Culture Media |
500 µL |
-80°C (Frozen) |
Dry Ice |
| Oil-based Extracts |
1 mL |
4°C (Refrigerated) |
Ice Pack |
Applications of Capsorubin Analysis
Food & Beverage
- Ensures purity and stability in natural colorants
- Supports regulatory compliance and nutritional labeling
- Assesses stability in processing and storage
- Detects adulteration in food products
Agriculture & Plant Research
- Studies carotenoid biosynthesis and metabolism
- Supports high-pigment cultivar selection
- Analyzes post-harvest retention in crops
Industrial & Environmental
- Optimizes natural pigment extraction for dyes
- Explores biosynthetic production methods
- Investigates antioxidant properties in various applications
Nutraceuticals & Supplements
- Standardizes functional ingredient content
- Assesses bioavailability and absorption
- Evaluates stability under storage conditions
Case. Designing a microbial factory suited for plant chloroplast-derived enzymes to efficiently and green synthesize natural products: capsanthin and capsorubin as examples
Background:
Plant chloroplast natural products (PCNPs) were analyzed.
The study aimed to design an innovative microbial factory to promote the heterologous synthesis of PCPNs, taking capsanthin and capsorubin as examples, by mimicking the chloroplast microenvironment in a microbial factory.
Samples:
E. coli DH5α was used for plasmid construction, and E. coli BL21 (DE3) was used for constructing carotenoid - producing strains.
Technical methods procedure:
Genes were optimized based on E. coli codon preference, obtained by PCR, and linked using infusion cloning technology. Plasmids were transformed into E. coli DH5α first and then into E. coli BL21(DE3).
The trxb gene in E. coli was knocked out using a two-plasmid system (pHCY-25A and pHCY-26D) designed for targeted genome editing.
Chloroplast transit peptides of proteins were predicted by SignalP - 6.0
The three - dimensional structures of enzymes in the carotenoid metabolic pathway were predicted by AlphaFold 3.
Carotenoids were analyzed by high - performance liquid chromatography (HPLC) after filtration.
Cytoplasmic and membrane proteins were purified by Ni²⁺ affinity chromatography
Protein concentration was measured by the Bradford method.
SDS - PAGE was performed with different concentrated gels for proteins of different sizes.
Results:
The accumulation of violaxanthin increased significantly and reduced upstream carotenoid accumulation.
Increasing the copies of CCS promoted the synthesis of violaxanthin and capsanthin.
Co - expressing chaperones provided a folding - promoting microenvironment for CCS.
Chloroplast - derived chaperones Cpn60α, Cpn60β, and Cpn20 were more effective in enhancing CCS catalytic performance, but all chaperones decreased ZEP activity.
Constructing an artificial homotrimer (AHT) of CCS and localizing it to the cell membrane increased the accumulation of capsanthin and capsorubin.
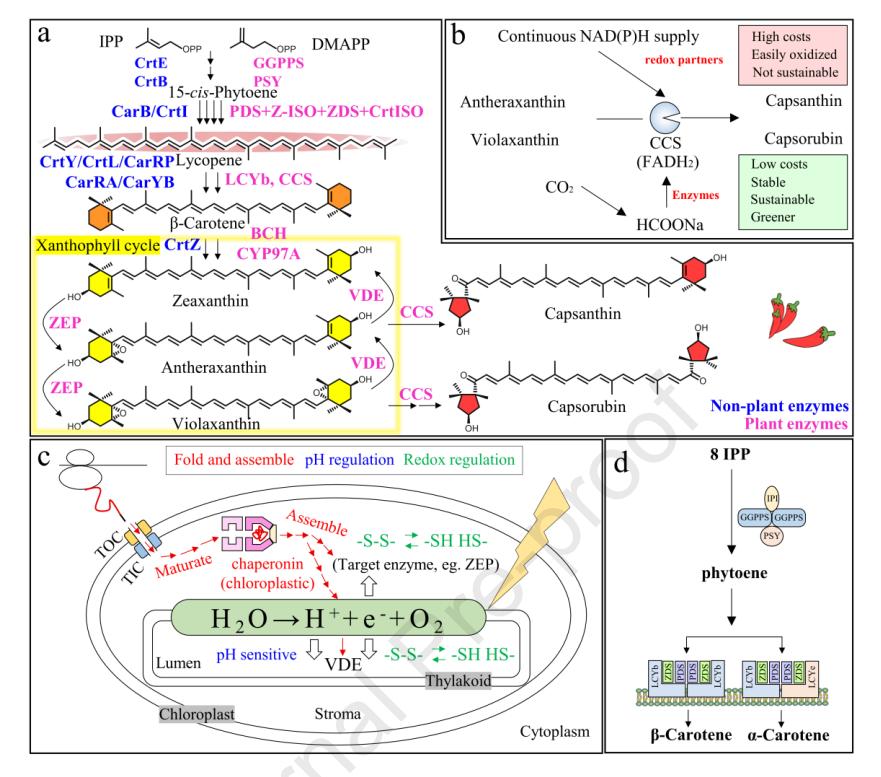 Overview and current obstacles of capsanthin/capsorubin biosynthesis.
Overview and current obstacles of capsanthin/capsorubin biosynthesis.
Reference
- Chen, H, et al. "Designing a microbial factory suited for plant chloroplast - derived enzymes to efficiently and green synthesize natural products: capsanthin and capsorubin as examples." Metabolic Engineering (2025). https://doi.org/10.1016/j.ymben.2025.01.005


 Agilent 1260 Infinity II HPLC (Figure from Agilent)
Agilent 1260 Infinity II HPLC (Figure from Agilent) Waters ACQUITY UPLC System (Figure from Waters)
Waters ACQUITY UPLC System (Figure from Waters) Agilent 7890B-5977B (Figure from Agilent)
Agilent 7890B-5977B (Figure from Agilent) SCIEX Triple Quad™ 6500+ (Figure from Sciex)
SCIEX Triple Quad™ 6500+ (Figure from Sciex)
 Overview and current obstacles of capsanthin/capsorubin biosynthesis.
Overview and current obstacles of capsanthin/capsorubin biosynthesis.