What are Cryptoxanthins?
Cryptoxanthin represents a significant class of carotenoid compounds, comprising a range of isomers and related derivatives. These are found in a variety of fruits and vegetables, as well as in certain algal plants. Cryptoxanthin is synthesised via a specific carotenoid synthesis pathway and plays a pivotal role in photoprotection and the regulation of hormone synthesis in plants. In addition, it constitutes a vital component of the human eye health maintenance and antioxidant defence system.
Analyzing cryptoxanthin is important for understanding plant physiology, biochemistry and human nutrition, and it provides valuable insights into the mechanisms by which plants adapt to light and regulate growth and development, as well as the metabolic pathways involved in human resistance to oxidative stress and maintenance of the visual system. Through the analysis and quantification of cryptoxanthin and its associated metabolites, along with the determination of their patterns of change in relation to particular plant growth stages, environmental stresses, or human health conditions, researchers can furnish a scientific basis for enhancing plant resilience, optimising the nutritional quality of crops, and safeguarding human health.
Cryptoxanthins Analysis Services by Creative Proteomics
Creative Proteomics offers a comprehensive range of cryptoxanthin assays. These programs precisely detect and measure cryptoxanthin in a range of samples by using state-of-the-art analytical techniques.
Comprehensive Identification of Cryptoxanthin: Precise detection, separation and quantification of cryptoxanthin in food, nutraceutical and plant tissue samples using high performance liquid chromatography (HPLC) for accurate measurement and relative content determination.
Detection of Target Cryptoxanthin: Liquid Chromatography-Mass Spectrometry (LC-MS) is utilized to accurately locate cryptoxanthin and measure the content of cryptoxanthin, while detecting potential impurities or isomers, thus ensuring the correctness and applicability of the results.
Cryptoxanthin Metabolic Tracer Assay: Provide stable isotope labeled cryptoxanthin, use advanced mass spectrometry to monitor the transformation trajectory of the labeled cryptoxanthin in the biological metabolic network, to determine the metabolic pathways involved, as well as the metabolism rate of cryptoxanthin in different tissues and physiological stages.
Techniques and Instrumentation for Cryptoxanthins Analysis
High Performance Liquid Chromatography (HPLC): Creative Proteomics used HPLC for the separation, detection and identification of cryptoxanthin. The HPLC system combine the separation power of a Thermo™ Scientific™ Vanquish HPLC or UHPLC system with precise fractionation for highly efficient purification to tailor cryptoxanthin characterisation or purification to suit the process.
 Thermo Scientific™ Vanquish™ (Figure from Thermo Fisher)
Thermo Scientific™ Vanquish™ (Figure from Thermo Fisher)
Liquid Chromatography-Mass Spectrometry (LC-MS): Creative Proteomics accurately detected and identified different species of cryptoxanthin by LC-MS. The Orbitrap Exploris™ 480 Mass Spectrometer provides LC-MS detection with market-leading resolution & and mass accuracy, selectivity & and spectral data quality, providing excellent sensitivity and selectivity for accurate identification of different species of cryptoxanthins.
 Orbitrap Exploris™ 480 (Figure from Thermo Fisher)
Orbitrap Exploris™ 480 (Figure from Thermo Fisher)
Workflow for Cryptoxanthins Analysis Service
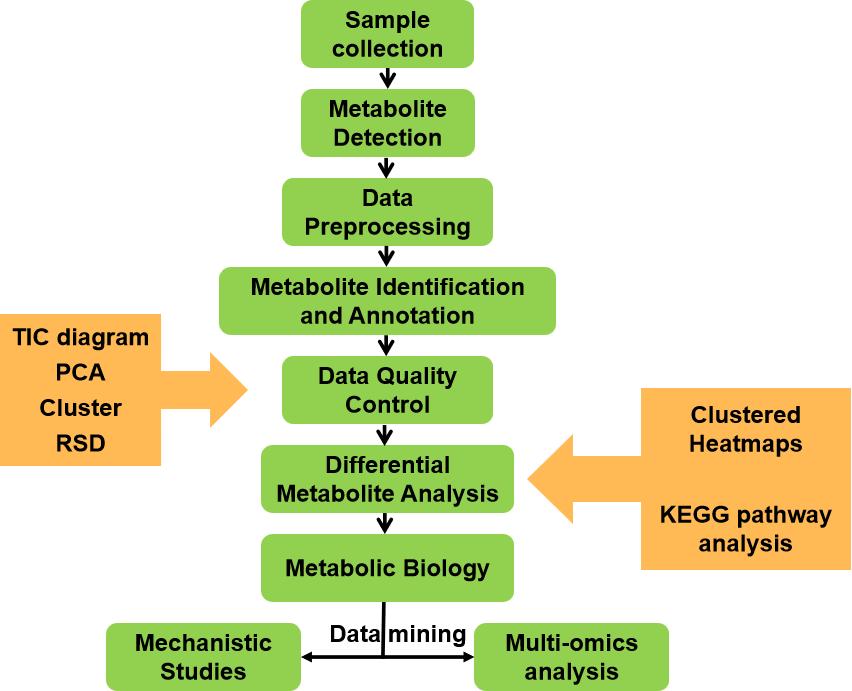
Why Choose Us?
- High Sensitivity & Accuracy: Achieve detection limits as low as ng/mL using LC-MS and HPLC, ensuring precise quantification of cryptoxanthin even in complex matrices.
- Comprehensive Detection: Orbitrap Exploris™ 480 provides <1 ppm mass accuracy for detailed cryptoxanthin profiling, including isomer and impurity identification.
- Metabolic Pathway Analysis: Using stable isotope-labeled cryptoxanthin, monitor metabolic transformations with high precision, tracking metabolic rates across various tissues and stages.
- Cutting-Edge Equipment: Thermo Vanquish HPLC ensures high resolution separation with up to 0.1% RSD for cryptoxanthin characterization.
- Optimized for Plant Research: Quantify cryptoxanthin levels in plants with high reproducibility and monitor changes due to growth or environmental stress.
- Health Impact Insights: Essential for evaluating cryptoxanthin's role in oxidative stress reduction and visual health maintenance.
Applications of Cryptoxanthins
Food quality testing and quality control: Cryptoxanthin analysis can be used to test the cryptoxanthin content in all kinds of cryptoxanthin-rich food products (e.g. citrus fruit products, pumpkin processed food, etc.), to ensure that the products comply with the nutritional labelling, and to help food enterprises to improve the quality of their products and to enhance their competitiveness in the market.
Drug R&D: Cryptoxanthin can be used as a single active ingredient or auxiliary ingredient in pharmaceutical preparations. Cryptoxanthin analysis can help to screen natural extracts containing high purity cryptoxanthin for drug development.
Crop quality improvement: For agricultural cultivation, it is important to cultivate cryptoxanthin-rich crop varieties. Cryptoxanthin analysis service can help researchers to analyse the cryptoxanthin content of different crop varieties or the same variety under different growth environments, screen out varieties with high cryptoxanthin content potential, and improve the quality of crops and the nutritional value of agricultural products through genetic breeding and other means.
Sample Requirements for Cryptoxanthins Assay
| Sample Types |
Minimum ample ize |
Biological Repeat |
| Plant Tissues |
Stem, leaf, flower tissue |
≥600 mg |
3-6 |
| bud, node, fruit tissue |
≥1 g |
| root tissue |
≥600 mg |
| Liquid Samples |
Root exudates |
5 mL |
| Fermentation broth, wine, tissue fluid, fruit juice |
5 mL |
| Honey, nectar, oil, extract |
500 μL |
| Specialty Samples |
Cultured samples, presence of liquid |
600 mg |
Case. Carotenoid Development and Physico-chemical Characteristics during Maturation of Red Fleshed Papaya Fruit (Carica papaya L.)
Background:
Carotenoid development of red fleshed papaya fruit was investigated.
The study aimed to understand the changes in carotenoid profiles and their relationship with fruit maturation, as well as the impact of on-tree vs postharvest ripening.
Samples:
Red fleshed papaya (C. papaya L.) fruits from hermaphrodite plants of the commercial Costa Rican hybrid "Pococí" were used.
Five fruits of each of three preharvest and four postharvest maturation stages were studied, along with five on-tree ripened fruits.
Technical methods procedure:
Morphological traits such as fruit weight, length, diameter were measured.
Texture of peel and pulp was determined using a texture analyzer.
Color analyses were carried out using a Colorflex instrument.
Chemical analyses for total soluble solids (TSS), pH and titratable aciditiy (TA) were done according to standard protocols.
Carotenoids were extracted from lyophilized mesocarp samples using a mixture of methanol, ethyl acetate and light petroleum.
HPLC-DAD coupled to mass spectrometry was used for carotenoid identification and quantification.
Results:
The morphological, physico-chemical and carotenoid changes during fruit maturation were analyzed and reported.
Differences in carotenoid profiles between early and late ripening stages were determined.
The comparison of on-tree and postharvest ripened fruits showed similar physico-chemical and carotenoid contents.
A ripening index based on TSS and pulp firmness was developed and correlated with carotenoid contents.
Specific carotenoids and their concentrations at different ripening stages were reported.
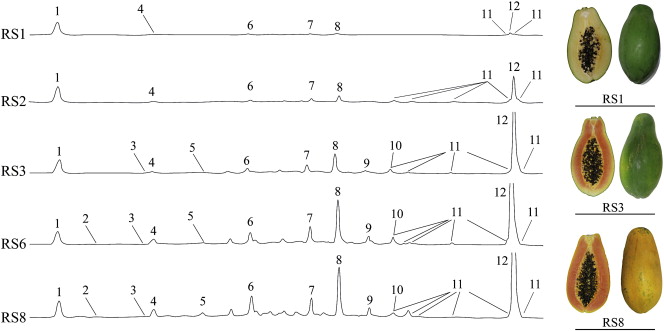 HPLC separation of carotenoids monitored at 450 nm and corresponding images of red papaya ripening stages.
HPLC separation of carotenoids monitored at 450 nm and corresponding images of red papaya ripening stages.
Reference
- Schweiggert R M, et al. "Carotenogenesis and physico-chemical characteristics during maturation of red fleshed papaya fruit (Carica papaya L.)." Food Research International (2011): 44. https://doi.org/10.1016/j.foodres.2011.01.029.


 Thermo Scientific™ Vanquish™ (Figure from Thermo Fisher)
Thermo Scientific™ Vanquish™ (Figure from Thermo Fisher) Orbitrap Exploris™ 480 (Figure from Thermo Fisher)
Orbitrap Exploris™ 480 (Figure from Thermo Fisher)
 HPLC separation of carotenoids monitored at 450 nm and corresponding images of red papaya ripening stages.
HPLC separation of carotenoids monitored at 450 nm and corresponding images of red papaya ripening stages.