What is Zeaxanthin?
Zeaxanthin, a xanthophyll carotenoid (C₄₀H₅₆O₂), is a critical antioxidant and light-filtering pigment found in plants, algae, and photosynthetic bacteria. As a key component of the human macular pigment, it protects retinal cells from oxidative damage and blue light exposure. Unlike animals, which must obtain zeaxanthin through diet, plants synthesize it via the carotenoid biosynthesis pathway, starting with geranylgeranyl diphosphate and involving enzymes such as lycopene β-cyclase (LycB) and zeaxanthin epoxidase (ZEP). Structurally, zeaxanthin differs from lutein in its double-bond arrangement, enhancing its role in photoprotection and oxidative stress mitigation.
Why Choose Us?
- High Sensitivity and Precision: Using LC-MS/MS and HPLC-DAD, we achieve detection limits as low as 0.1 ng/mL, ensuring accurate detection even at trace levels.
- Comprehensive Metabolite Detection: We identify zeaxanthin's metabolites and precursors, such as lutein and violaxanthin, with 98%+ accuracy, offering a complete metabolic profile.
- High-Throughput Capacity: Our systems process up to 500 samples per day, ensuring fast turnaround without compromising data quality.
- Superior Resolution and Reproducibility: With >10,000 plates in HPLC/UPLC, we provide precise separation and reproducible results across various sample types.
- Quick Turnaround: Results are delivered in 10–14 business days, ideal for fast-paced research or commercial projects.
Zeaxanthin Analysis Services by Creative Proteomics
- Zeaxanthin Quantification – Accurate measurement of zeaxanthin content in various samples using advanced chromatographic and spectrometric techniques.
- Zeaxanthin Profiling – Comprehensive analysis of zeaxanthin and its related carotenoids to study metabolic pathways and biosynthesis.
- Zeaxanthin Stability Testing – Evaluation of zeaxanthin stability under different environmental conditions (e.g., temperature, light exposure, pH).
- Zeaxanthin Distribution Analysis – Detection and quantification of zeaxanthin in different biological matrices, including plant tissues, food products, and supplements.
- Comparative Zeaxanthin Analysis – Comparative studies of zeaxanthin levels across different plant varieties, food products, or experimental conditions.
- Customized Zeaxanthin Analysis – Tailored analytical solutions to meet specific research or industrial requirements, including method development and validation.
List of Detected Zeaxanthin and Related Metabolites
| Category |
Detected Compounds |
| Carotenoids |
Zeaxanthin, Lutein, β-Carotene, α-Carotene, Cryptoxanthin |
| Zeaxanthin Derivatives |
Zeaxanthin Epoxide, Violaxanthin, Neoxanthin |
| Oxidative Metabolites |
Zeaxanthin Quinone, Apocarotenoids |
| Precursor Carotenoids |
Phytoene, Phytofluene, Lycopene, Astaxanthin |
Methods and Instrumentation for Zeaxanthin Assay
HPLC-DAD – Agilent 1200 Series HPLC, detects zeaxanthin at 450 nm.
UPLC-PDA – Waters ACQUITY UPLC, enhances separation with higher resolution.
LC-MS/MS – AB SCIEX Triple Quadrupole 6500+, highly sensitive quantification.
GC-MS – Agilent 7890B GC & 5977A MS, detects derivatized carotenoids.
 Agilent 1260 Infinity II HPLC (Figure from Agilent)
Agilent 1260 Infinity II HPLC (Figure from Agilent)
 Waters ACQUITY UPLC System (Figure from Waters)
Waters ACQUITY UPLC System (Figure from Waters)
 Agilent 7890B-5977B (Figure from Agilent)
Agilent 7890B-5977B (Figure from Agilent)
 SCIEX Triple Quad™ 6500+ (Figure from Sciex)
SCIEX Triple Quad™ 6500+ (Figure from Sciex)
Workflow for Zeaxanthin Analysis Service
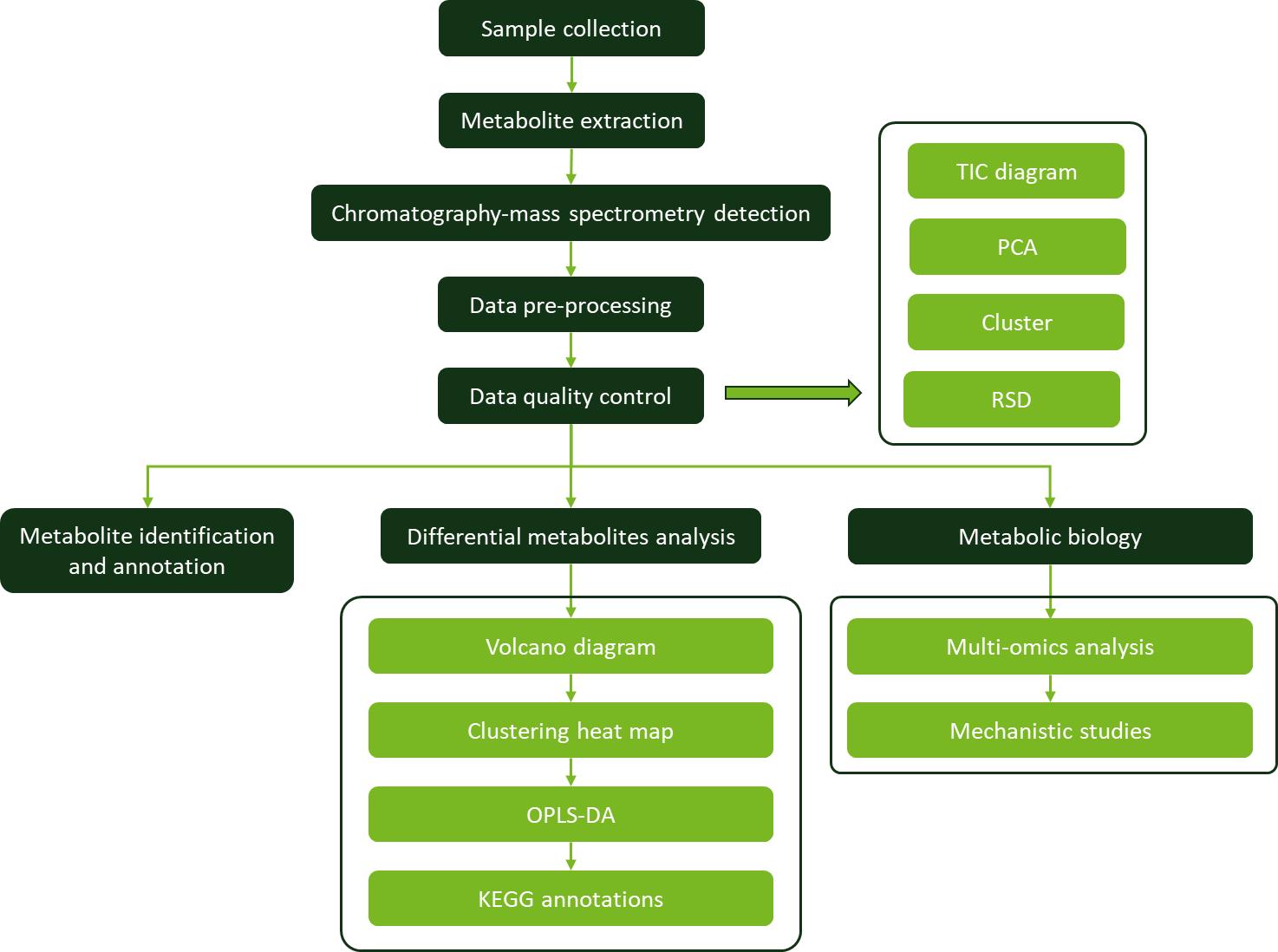
Sample Requirements for Zeaxanthin Assay
| Sample Types |
Minimum Sample Size |
Biological Repeat |
| Plant Tissues |
Stem, leaf, flower tissue |
≥1 g |
3-6 |
| bud, node, fruit tissue |
≥1 g |
| root tissue |
≥1 g |
| Liquid Samples |
Root exudates |
10 mL |
| Fermentation broth, wine, tissue fluid, fruit juice |
10 mL |
| Honey, nectar, oil, extract |
500 μL |
| Specialty Samples |
Cultured samples, presence of liquid |
600 mg |
Applications of Zeaxanthin Analysis
Case. Melatonin alleviates drought stress and increases lutein and zeaxanthin industrial pigments in African marigold by some physiological regulations
Background:
The effects of drought stress on African marigold and the role of melatonin in alleviating stress were investigated.
The study aimed to explore how melatonin influences the agro-morphological traits, biochemical processes, and phytochemical profiles of African marigold under different drought stress levels.
Samples:
African marigold (Tagetes erecta L.) seeds of the F1-yellow dwarf variety were planted in pots with specific soil composition in the research greenhouse of Ilam University.
Plants were subjected to 4 levels of drought stress (100%, 80%, 60%, 40% FC) and 3 levels of melatonin treatment (0, 100, 200 µmol).
Technical methods procedure:
Morphological traits including plant height, fresh and dry weights of shoots, roots, and flowers, as well as flower diameter were measured using rulers, digital scales, and calipers.
Leaf surface area was determined using a digital area meter.
Chlorophyll and carotenoid contents were analyzed by grinding leaf samples in acetone and measuring absorbance at specific wavelengths through a spectrophotometer.
Lutein concentration was measured by powdering flower samples, mixing them with solvents, filtering, centrifuging, and measuring absorbance at 446 nm.
Zeaxanthin content was determined using HPLC with a designated column and mobile phase.
Free proline content was measured by mixing powdered leaves with liquid nitrogen and reagents, followed by centrifugation, reaction, and absorbance measurement at 520 nm.
Electrolyte leakage was measured using a digital EC meter.
Hydrogen peroxide content was analyzed by the potassium iodide reaction method and absorbance measurement at 390 nm.
Antioxidant activity was measured by adding DPPH solution to extracts and calculating the free radical percentage based on absorbance at 517 nm.
Catalase, peroxidase, and superoxide dismutase enzyme activities were measured by their respective reaction methods and monitoring absorbance changes.
GABA was quantified by homogenizing leaf tissue, extracting, filtering, injecting into HPLC, and using a fluorometric detector.
Protein content was measured by grinding leaf tissue in buffer, centrifuging, and using a spectrophotometer with a standard curve.
Results:
The changes in agro-morphological traits under drought stress and the improvement effects of melatonin treatments were analyzed and reported.
The variations in chlorophyll, carotenoids, lutein and zeaxanthin contents with stress levels and melatonin treatments were determined.
The effects of melatonin on hydrogen peroxide, superoxide, electrolyte leakage, proline, protein content, DPPH activity and antioxidant enzyme activities were analyzed.
The relationship between melatonin treatment and GABA content was investigated.
Melatonin treatments were found to enhance plant growth, increase pigment contents, improve antioxidant capacity and osmolality under drought stress.
The 200 µmol melatonin treatment showed better performance in most aspects.
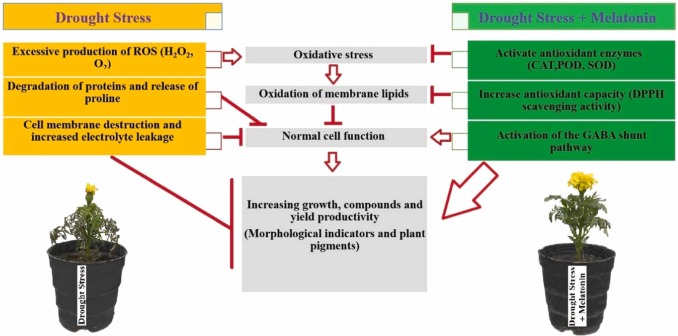 The multiple mechanisms of melatonin-induced alleviation of drought sress responses in plants
The multiple mechanisms of melatonin-induced alleviation of drought sress responses in plants
Reference
- Meisam Mohammadi, et al. "Melatonin alleviates drought stress and increases lutein and zeaxanthin industrial pigments in African marigold by some physiological regulations." Journal of Industrial Crops and Products (2024): 120089. https://doi.org/10.1016/j.indcrop.2024.120089


 Agilent 1260 Infinity II HPLC (Figure from Agilent)
Agilent 1260 Infinity II HPLC (Figure from Agilent) Waters ACQUITY UPLC System (Figure from Waters)
Waters ACQUITY UPLC System (Figure from Waters) Agilent 7890B-5977B (Figure from Agilent)
Agilent 7890B-5977B (Figure from Agilent) SCIEX Triple Quad™ 6500+ (Figure from Sciex)
SCIEX Triple Quad™ 6500+ (Figure from Sciex)

 The multiple mechanisms of melatonin-induced alleviation of drought sress responses in plants
The multiple mechanisms of melatonin-induced alleviation of drought sress responses in plants