Phytosterols, often referred to as plant sterols, represent a heterogeneous ensemble of natural compounds that pervade the intricate matrix of plant cell membranes. These structural analogs to cholesterol confer vital biomechanical stability to cell membranes and tangibly impact membrane fluidity. The biological relevance of phytosterols transcends their structural fortitude. Emerging scientific discourse suggests that phytosterols proffer discernible cholesterol-lowering attributes, thereby substantiating their potential role in cardiovascular health. Furthermore, the nuanced biochemical repertoire of phytosterols has been postulated to encompass an arsenal of bioactivities, encompassing anti-inflammatory, antioxidative, and even anti-carcinogenic attributes, thereby accentuating their pertinence within the biomedical realm.
The analytical scrutiny of phytosterols transcends conventional academic curiosity, birthing profound ramifications for diverse industries including functional foods, nutraceuticals, pharmaceuticals, and agriculture. Creative Proteomics, an exemplar of scientific precision, extends its purview through the Phytosterols Analysis Service, thereby facilitating an unprecedented molecular understanding of these enigmatic compounds.
Creative Proteomics' Multifaceted Phytosterols Analysis Service
The Phytosterols Analysis Service offered by Creative Proteomics embodies an intricate tapestry of analytical dimensions, each designed to unravel specific facets of phytosterols' complexity. The pillars of this service include:
Comprehensive Profiling: A panoramic exploration of phytosterol composition across diverse samples, unearthing their structural diversity and relative abundance.
Quantitative Precision: Accurate quantification of phytosterols, facilitating precise dose-response evaluations and comparative analyses.
Structural Isomer Differentiation: Discrimination of phytosterol isomers, unraveling the subtle structural variations and their potential implications in molecular interactions.
Metabolic Trajectory Mapping: Deciphering the intricate metabolic pathways of phytosterols, shedding light on their transformations and interactions within intricate biochemical networks.
Phytosterols Analytical Techniques
Thermo Scientific Q Exactive HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer: This pinnacle of mass spectrometry technology excels in comprehensive phytosterol profiling. Equipped with a precision quadrupole and a high-field Orbitrap analyzer, the Q Exactive HF-X offers unmatched resolution, precise mass accuracy, and an expansive dynamic range. It is adept at dissecting complex matrices, deciphering structural nuances, and quantifying trace constituents. The Q Exactive HF-X is an optimal choice for exploring phytosterol diversity and abundance.
Agilent 6495B Triple Quadrupole LC/MS System: Recognized for its robustness and quantitative precision, the Agilent 6495B is designed for targeted phytosterol quantitation. Leveraging triple quadrupole technology, it excels in the precise determination of specific phytosterols within complex samples. The capacity for executing multiple reaction monitoring (MRM) assays enhances its sensitivity and specificity, rendering it an ideal choice for quantifying trace phytosterols within a variety of matrices.
Waters SYNAPT G2-Si High Definition Mass Spectrometer: Integrating quadrupole ion mobility separation with high-resolution mass spectrometry, the SYNAPT G2-Si is proficient in structural elucidation. The SYNAPT G2-Si introduces an additional layer of specificity to phytosterol analysis, distinguishing isomers based on gas-phase mobility. Its strength lies in unveiling the intricate structural features of phytosterols, making it suitable for unraveling isomeric patterns and elucidating complex mixtures.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Phytosterols Analyzed (including but not limited to)
| Group |
Phytosterols and Related Compounds |
| Sterols |
Beta-Sitosterol, Campesterol, Stigmasterol, Delta-5-Avenasterol, Delta-7-Stigmasterol, Brassicasterol, Cycloartenol, 24-Methylenecholesterol, Obtusifoliol, Fucosterol, Sitostanol, Delta-7-Avenasterol, 24-Methylenelophenol, 24-Methylenelanosterol, Stigmastanol |
| Stanol Esters |
Sitostanol Esters, Campestanol Esters, Brassicastanol Esters |
| Acylated Sterols |
Acylated Steryl Glucosides, Acylated Steryl Glycosides, Acylated Steryl Oligosaccharides, Acylated Steryl Monoglycosides, Acylated Steryl Diglycosides |
| Other Sterol Derivatives |
Avenasterol, 24-Ethylcholesterol, 22-Dihydrobrassicasterol, 24-Ethylcoprostanol, Clerosterol, Gramisterol |
| Miscellaneous Phytosterols |
Lanosterol, Dihydrolanosterol, Citrostadienol, Citrostadienol Acetate, Lathosterol, Obtusifoliol |
Sample Requirements for Phytosterols Assay
| Sample Types |
Minimum Sample Size |
| Plant Samples |
Roots, stems and leaves, floral parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/young plants, rhizosphere soil, root exudates. |
50 mg - 1 g |
| Animal Samples |
Serum/Plasma |
200-500 μL |
| Tissues |
20-100 mg |
| Urine/Feces |
1-2 mL / 100 mg |
| Cell Samples |
Cells and Culture |
106 - 108 cells |
Case 1. Exploring Marine Macrophyte Lipidomics: Innovative Mass Spectrometry Analysis and Bioactive Discoveries
Background:
Phytosterols, natural compounds with structural similarities to cholesterol, are widely distributed in plant sources and have garnered significant attention for their potential health benefits. These bioactive substances are especially prevalent in edible oils, including corn, soybean, and rapeseed oils. Research has indicated that phytosterols can play a pivotal role in reducing cholesterol absorption and potentially contribute to the prevention of various diseases such as cancer, cardiovascular disorders, and metabolic ailments.
Samples:
The study encompassed an array of plant-derived products, with a primary focus on edible oils renowned for their phytosterol content. Notably, oilseed varieties like corn, soybean, and rapeseed were examined due to their high phytosterol levels. In addition, the investigation extended to include wheat germ oil, characterized by exceptionally elevated phytosterol concentrations. The choice of these samples aimed to represent a diverse spectrum of plant sources and highlight the varying phytosterol profiles across different oil types. These oils serve as vital dietary components and are widely utilized in cooking and food preparation, making them essential subjects for understanding phytosterol distribution and transformations.
Methods:
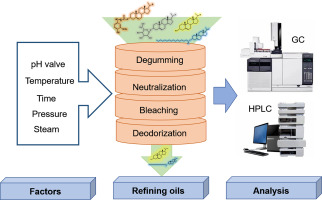
Phytosterols Separation and Extraction: Different chromatographic techniques (CC, TLC, SPE) were employed to separate and extract free phytosterols (FPs) and phytosteryl esters (PEs) from the complex matrix of edible oils. Hydrolysis and silylation processes were used to prepare samples for analysis.
Gas Chromatography (GC) Analysis: GC, coupled with Flame Ionization Detection (FID) or Mass Spectrometry (MS), was utilized for accurate separation and quantification of phytosterols. Derivatization methods, such as silylation, enhanced the analysis of non-silylated phytosterols.
High-Performance Liquid Chromatography (HPLC) Analysis: HPLC, particularly reversed-phase HPLC (RP-HPLC), was employed to separate phytosterols and their derivatives. UV, DAD, ELSD, or RI detection methods were used for quantitation. HPLC-MS was utilized for structural identification and quantification of various phytosterol species.
Results
Phytosterol Distribution in Edible Oils: Vegetable oils, especially corn and wheat germ oils, exhibited high phytosterol content compared to other plant-based products. Sitosterol, campesterol, and stigmasterol were vital components. Content variations were observed due to oil type, source, genetics, and refining processes.
Changes During Refining: The refining process led to progressive phytosterol loss. Degumming, neutralization, bleaching, and deodorization steps all contributed to phytosterol reduction, influenced by factors like polarity, acid/base hydrolysis, and competitive adsorption with other components.
Oxidation During Storage: Phytosterols were susceptible to oxidation during storage, affected by temperature, moisture, and oxygen levels. Phytostanols showed relative stability, while FPs and PEs demonstrated oxidation and degradation, impacting their overall content and quality.
Analytical Techniques: GC and HPLC played key roles in quantifying and characterizing phytosterols. GC provided sensitive separation, while HPLC offered advantages in analyzing thermally unstable compounds. HPLC-MS and GC-MS enabled identification and quantification of phytosterol derivatives.
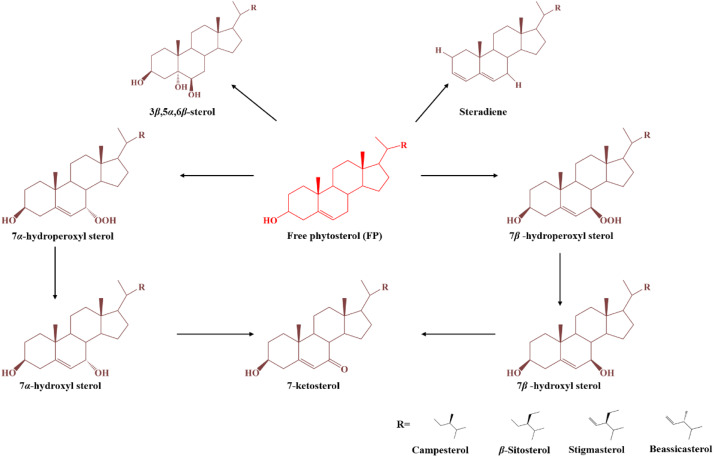 Molecular conformational change of phytosterols during the frying processes.
Molecular conformational change of phytosterols during the frying processes.
In conclusion, the study investigated phytosterols' presence, changes during refining and storage, and analytical methods. Vegetable oils emerged as rich sources, while refining and storage processes affected phytosterol content and quality. Advanced chromatographic techniques enabled accurate analysis and quantification of phytosterols and their derivatives.
Reference
- Bai, Ge, Chuanguo Ma, and Xiaowei Chen. "Phytosterols in edible oil: Distribution, analysis and variation during processing." Grain & Oil Science and Technology 4.1 (2021): 33-44.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
 Molecular conformational change of phytosterols during the frying processes.
Molecular conformational change of phytosterols during the frying processes.