What are Ketones Compounds?
Ketone compounds are organic molecules characterized by a carbonyl group (C=O) bonded to two carbon atoms. This unique structure makes them highly reactive and versatile, playing crucial roles in both biological systems and industrial applications. In biological contexts, ketones are essential intermediates in metabolic processes like fatty acid oxidation and carbohydrate metabolism, often serving as energy substrates. For instance, ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone are vital energy sources during fasting or ketogenic states. In industrial settings, ketones are widely used as solvents, chemical synthesis precursors, and in the production of plastics, pharmaceuticals, and agrochemicals.
Why Choose Ketone Compound Analysis?
Analyzing ketone compounds delivers critical insights for various applications:
- Metabolic Research: Monitor ketone levels to explore metabolic diseases, energy production pathways, and the effects of dietary interventions like ketogenic diets.
- Drug Development: Identify ketones as biomarkers or utilize them as chemical scaffolds for new drug discovery.
- Food and Beverage Quality Control: Ensure product authenticity and quality by detecting ketone-based flavor compounds.
- Environmental Monitoring: Assess ecological impact and ensure regulatory compliance by identifying and quantifying ketone pollutants.
Creative Proteomics offers advanced ketone analysis services, enabling researchers and industries to achieve precise, actionable insights to support innovation, quality assurance, and informed decision-making.
Ketones Compounds Analysis Offered by Creative Proteomics
Creative Proteomics provides a comprehensive suite of ketone compound analysis services. Our services include:
Quantitative Analysis of Ketone Compounds: Accurately measure the concentration of various ketone compounds in diverse sample types. Generate high-quality, reproducible data essential for metabolic profiling and industrial quality control.
Qualitative Identification of Ketone Compounds: Identify and confirm the presence of ketone compounds using high-resolution mass spectrometry techniques. Provide detailed molecular characterization for structural insights.
Pathway Analysis and Interpretation: Map ketone compounds within biological pathways to uncover their roles in metabolic processes. Deliver customized pathway visualization and interpretation reports.
Environmental and Industrial Ketone Analysis: Detect and quantify ketone pollutants in environmental samples such as water, soil, and air. Support industrial production with impurity profiling and product quality assessment.
Customized Solutions: Tailor analytical workflows to address unique research questions or specific industrial challenges. Offer specialized support for cross-disciplinary projects integrating ketone analysis with other omics studies.
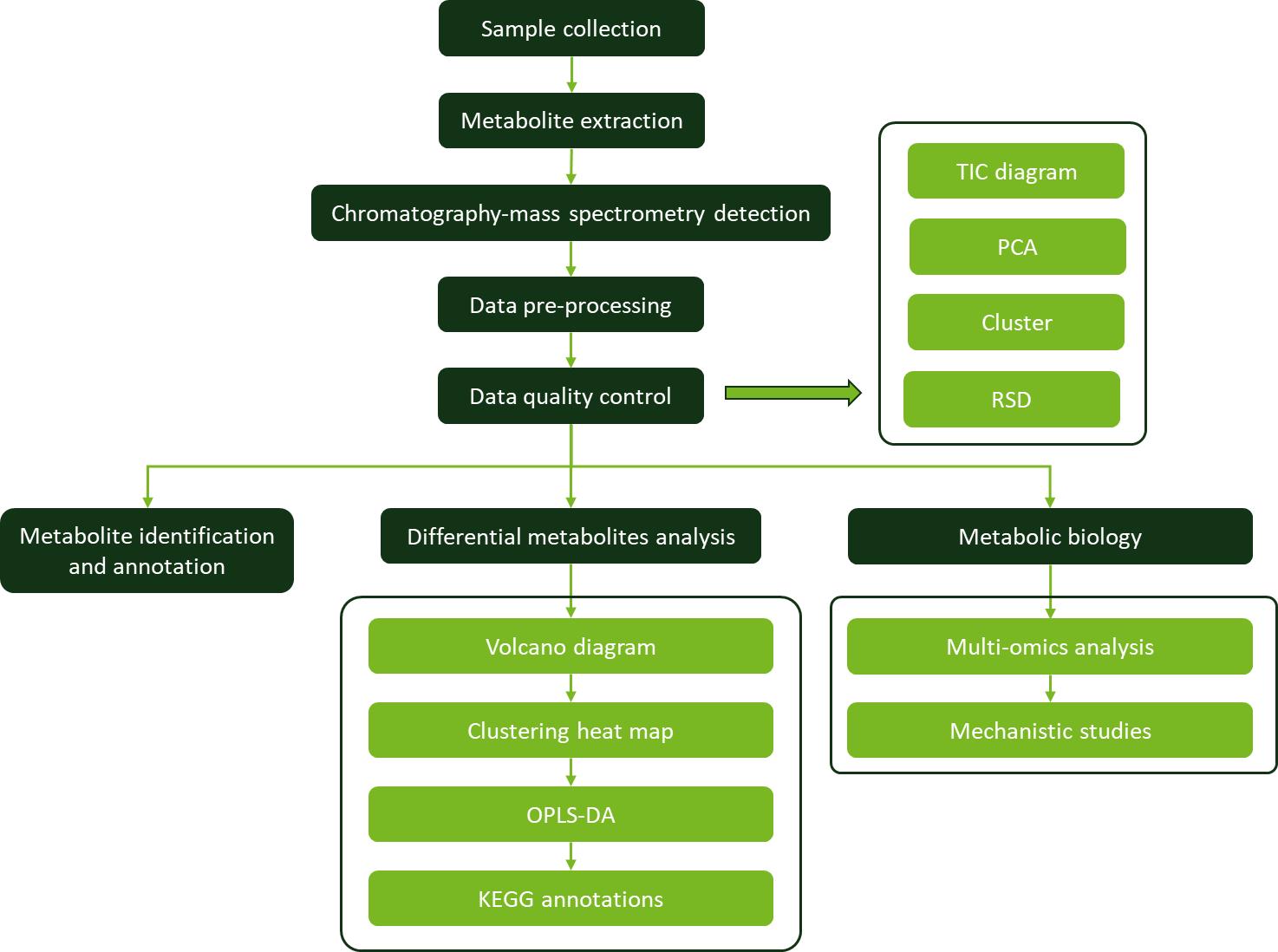 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Technological Platforms for Ketones Compounds Analysis
Gas Chromatography-Mass Spectrometry (GC-MS)
Instrument: Agilent 7890B GC coupled with a 5977B MS detector.
Application: Ideal for volatile and semi-volatile ketones.
Liquid Chromatography-Mass Spectrometry (LC-MS)
Instrument: Thermo Scientific™ Q Exactive™ HF Orbitrap LC-MS.
Application: Suitable for non-volatile and polar ketones.
High-Resolution Mass Spectrometry (HRMS)
Instrument: SCIEX TripleTOF™ 6600.
Application: For highly sensitive and accurate ketone compound profiling.
Tandem Mass Spectrometry (MS/MS)
Instrument: AB Sciex 5500 QTRAP®.
Application: Facilitates structural elucidation of ketone molecules.
List of Ketone Compounds We Can Analyze
| Category |
Ketone Compounds |
| Simple Ketones |
Acetone, Methyl Ethyl Ketone, Cyclohexanone, 2-Butanone |
| Aromatic Ketones |
Benzophenone, Acetophenone, Fluorenone, Anthraquinone |
| Diketones |
Diacetyl, Acetylacetone, 2,3-Pentanedione, 1,4-Benzoquinone |
| Hydroxy Ketones |
Hydroxyacetone, Hydroxycyclohexanone, 4-Hydroxy-2-butanone |
| Keto Acids |
Pyruvic Acid, Acetoacetic Acid, Oxaloacetic Acid, Levulinic Acid |
| Keto Sugars |
Fructose, Sorbose, Tagatose |
| Cyclic Ketones |
Cyclopentanone, Cyclohexanone, Camphor |
| Halogenated Ketones |
Trichloroacetone, Bromopentanone, Chlorocyclohexanone |
| Specialty Ketones |
β-Keto Esters, β-Keto Amides, Keto Steroids |
| Environmental Ketones |
Methyl Isobutyl Ketone, Acetone Derivatives, Ketone Pollutants |
Sample Requirements for Ketones Compounds Assay
| Sample Type |
Minimum Amount Required |
Storage Conditions |
| Animal Tissues |
≥ 100 mg |
-80°C |
| Plant Tissues |
≥ 200 mg |
-80°C |
| Biofluids |
≥ 100 µL |
-80°C, collected in EDTA tubes |
| Cell Samples |
≥ 1 × 10⁶ cells |
Flash-frozen in liquid nitrogen |
| Environmental Samples |
≥ 100 mg or ≥ 100 µL |
Store at 4°C or freeze at -20°C |
Applications of Ketones Compounds Analysis
Pharmaceutical Development:
- Optimize drug design using ketone-based molecular scaffolds.
- Analyze ketone intermediates and derivatives in synthetic pathways.
Food and Beverage Industry:
- Authenticate and quality-assess food products by detecting ketone-based flavor and aroma compounds.
- Control fermentation processes by monitoring ketone byproducts.
Environmental Monitoring:
- Detect ketone pollutants in air, water, and soil samples to assess ecological impacts.
- Ensure compliance with environmental regulations by quantifying hazardous ketones.
Industrial Quality Control:
- Validate the purity and consistency of ketone-containing raw materials and finished products.
- Identify and quantify impurities or byproducts in chemical manufacturing processes.
Biotechnology and Synthetic Biology:
- Evaluate ketone precursors and products in engineered metabolic pathways.
- Integrate ketone analysis with multi-omics data to enhance pathway optimization.
Background
This section describes a detailed study involving the synthesis, stability, and quantification of acetoacetate (AcAc) and β-hydroxybutyrate (βOHB) in biological samples, such as serum and liver tissues. The work is centered on the use of LC-MS for the accurate quantification of ketone bodies and the investigation of their stability under different storage conditions. The research aims to refine analytical methods for studying metabolic processes, especially in relation to fasting and ketogenesis in mice.
Materials & Methods
Chemicals and Materials:
- Reagents: Methanol, acetonitrile, water (LC-MS grade), (S)-2-Hydroxybutyric acid, acetic acid, and others were sourced from Thermo Fisher Scientific and Sigma-Aldrich.
- Internal Standards: [3,4,4,4-D4]βOHB, [U–13C4]AcAc were used for quantification purposes.
- Special Reagents: Triphenylphosphine (TPP), sodium borodeuteride (NaBD4), and others for chemical synthesis processes.
Synthesis of AcAc, [U–13C4]AcAc (I.S.) and 4-Hydroxybutyrate (4-OHB):
- The synthesis of AcAc and related compounds was achieved by base-catalyzed hydrolysis of precursors like ethyl-acetoacetate and ethyl-[U–13C4]AcAc.
- Products were neutralized, stored at -80°C, and used in subsequent experiments.
AcAc Stability Testing:
- AcAc samples were stored under varying conditions (room temperature, refrigeration, freezing, etc.) to test their stability over time.
- βOHB was added as a stabilizing agent to monitor the degradation of AcAc.
- Serum from wild-type mice was pooled, spiked with labeled βOHB, and subjected to the same storage conditions.
Biospecimen Preparation:
- Wild-type mice were used for serum and tissue collection.
- Mice were fasted or given specific treatments (e.g., antisense oligonucleotide injections) to induce ketogenesis or modify specific metabolic pathways.
- Blood and liver tissues were processed for analysis.
Quantification of [U–13C4]AcAc Internal Standard (I.S.):
- The concentration of synthesized [U–13C4]AcAc was quantified by reducing it to βOHB using NaBD4 and then measuring via mass spectrometry.
- Ion suppression effects were minimized by using Dowex for desalting.
Sample Extraction and Analysis:
- Serum samples were extracted using a cold ACN/MeOH solution, followed by centrifugation.
- Extracts were analyzed by UPLC-MS/MS with PRM (Parallel Reaction Monitoring) mode for ketone quantification.
- High-throughput methods for serum extraction were also implemented.
Instrumentation:
- UPLC systems were optimized for the analysis of ketones using Atlantis T3 and Cortecs UPLC T3 columns.
- Thermo Q Exactive Plus MS with heated electrospray ionization was used for the quantification of ketone bodies.
- Data analysis involved PRM mode with specified m/z transitions for AcAc and βOHB.
Calibration, Quantification, and Validation:
- Calibration curves for AcAc and βOHB were constructed using standard solutions and internal standards.
- Precision and recovery were validated through repeated injections and spike recovery experiments.
- Linearity, limit of detection (LOD), and limit of quantification (LOQ) were also assessed.
Statistical Analysis:
- Statistical analysis was performed using GraphPad Prism, where normality tests and various statistical methods were applied based on experimental design.
Results
Separation and Identification of β-Hydroxybutyrate (βOHB) and Acetoacetate (AcAc)
The analysis of a mouse serum extract spiked with [3,4,4,4-D4]βOHB was conducted using an Atlantis T3 C18 column coupled with a Q-Exactive Plus MS system in negative ionization mode. The serum extract exhibited peak shoulder for βOHB, indicating possible co-elution with other βOHB isomers, such as 3-hydroxyisobutyrate (3-HIB), 4-hydroxybutyrate (4-OHB), and 2-hydroxybutyrate (2-OHB). Improved separation was achieved using a Cortecs UPLC T3 column, with βOHB and 3-HIB identified by retention time, m/z, and MS/MS spectra. The study revealed a marked difference in the relative contributions of βOHB, 3-HIB, and 2-OHB in the serum of ketogenesis insufficient mice, where 64.3% of the m/z 103.0401 signal originated from 3-HIB and 2-OHB, compared to 15.3% in control mice.
Acetoacetate Stability
Acetoacetate (AcAc) demonstrated stability in water at various temperatures, with only a significant decay observed at room temperature after 28 days. In the extraction solution of AcN/MeOH/water (2:2:1), AcAc stability decreased more rapidly, with a 21.8% loss at room temperature within 6 hours. However, AcAc remained stable at temperatures ≤ 4°C for up to 35 days. In serum from fasted mice, AcAc was highly unstable at +37°C, showing a 65.9% loss in 6 hours. When stored at −80°C or in liquid nitrogen (LN2), AcAc stability was preserved. Moreover, freeze-thaw cycles did not significantly affect the stability of AcAc in both synthesized and endogenous forms.
Development of Internal Standards for Quantification
To improve quantification accuracy, a [U–13C4]AcAc internal standard was synthesized. This standard facilitated the reduction of AcAc to βOHB with a high yield, allowing precise quantification of both compounds. The UPLC-MS/MS method for AcAc and βOHB analysis exhibited excellent linearity (R² ≥ 0.997) with low limits of detection (LOD) and quantification (LOQ), well below the concentrations typically found in biological samples. Precision studies showed good reproducibility, with inter-sample precision ≤ 6.5% and recovery rates ranging from 95.1% to 110.9%. In vivo, fasting in wild-type mice led to incremental increases in βOHB and AcAc levels, with βOHB increasing more than AcAc. In ketogenesis insufficient mice, βOHB levels were significantly lower, showing a blunted response to fasting.
Discrimination of βOHB Enantiomers and Structural Isomers
The derivatization of βOHB with S-PMP enabled effective separation of D- and L-βOHB enantiomers in serum. Analysis showed that the D-βOHB enantiomer was predominant in the serum of control mice, while in ketogenesis insufficient mice, D-βOHB contributed only 65.4%. Additionally, 3-HIB was found exclusively as the L-enantiomer in both control and ketogenesis insufficient mice, while 2-OHB was almost entirely L-enantiomeric in both groups.
 Application of novel UPLC-MS/MS method to quantify AcAc and βOHB concentrations in serum and liver tissue extracts
Application of novel UPLC-MS/MS method to quantify AcAc and βOHB concentrations in serum and liver tissue extracts
 MS/MS detection, identification and UPLC separation of DL-βOHB enantiomers and their structural isomers 2-OHB, 3-HIB, and 4-OHB.
MS/MS detection, identification and UPLC separation of DL-βOHB enantiomers and their structural isomers 2-OHB, 3-HIB, and 4-OHB.
Reference
- Puchalska, Patrycja, et al. "Determination of ketone bodies in biological samples via rapid UPLC-MS/MS." Talanta 225 (2021): 122048.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Application of novel UPLC-MS/MS method to quantify AcAc and βOHB concentrations in serum and liver tissue extracts
Application of novel UPLC-MS/MS method to quantify AcAc and βOHB concentrations in serum and liver tissue extracts MS/MS detection, identification and UPLC separation of DL-βOHB enantiomers and their structural isomers 2-OHB, 3-HIB, and 4-OHB.
MS/MS detection, identification and UPLC separation of DL-βOHB enantiomers and their structural isomers 2-OHB, 3-HIB, and 4-OHB.