What are Flavanonols?
Flavanonols are a subclass of flavonoids, which are a group of plant secondary metabolites known for their diverse biological activities and potential health benefits. Flavanonols are structurally similar to flavonols but have a different carbon skeleton arrangement. They are characterized by a 3-hydroxy-2-phenylchromen-4-one structure. Some common flavanonols include taxifolin (also known as dihydroquercetin), astilbin, and engeletin.
These compounds are found in various plant-based foods such as fruits, vegetables, tea, and wine. Flavanonols possess antioxidant properties and have been studied for their potential role in reducing oxidative stress, inflammation, and promoting overall health. They may also have potential applications in pharmaceuticals, nutraceuticals, and functional foods due to their bioactive properties.
Creative Proteomics offers tailored solutions to meet the requirements of distinct flavanonol analysis.
Flavanonols Analysis at Creative Proteomics
- Flavanonol Identification: Comprehensive identification of flavanonols in various samples using advanced analytical techniques.
- Flavanonol Quantification: Accurate quantification of flavanonols in samples of interest through validated analytical methods.
- Flavanonol Profiling: Profiling of flavanonol composition in complex samples to understand their distribution and abundance.
- Flavanonol Structure Elucidation: Structural elucidation of flavanonol compounds using spectroscopic and chromatographic techniques.
- Flavanonol Bioactivity Assessment: Evaluation of the bioactive properties of flavanonols, including antioxidant, anti-inflammatory, and anticancer activities.
Flavanonol Metabolism Analysis Techniques
High-Performance Liquid Chromatography (HPLC): Our HPLC systems, such as Agilent 1260 Infinity II HPLC, enable high-resolution separation of flavanonol compounds in complex mixtures.
Liquid Chromatography-Mass Spectrometry (LC-MS): Utilizing instruments like the Thermo Scientific™ Q Exactive™ HF-X Hybrid Quadrupole-Orbitrap™ Mass Spectrometer coupled with liquid chromatography, we achieve sensitive detection and structural elucidation of flavanonols with unparalleled accuracy.
Gas Chromatography-Mass Spectrometry (GC-MS): With equipment such as the Agilent 7890B Gas Chromatograph coupled with the Agilent 5977A Mass Selective Detector, we analyze volatile flavanonol derivatives and precursors with exceptional separation efficiency.
Nuclear Magnetic Resonance (NMR) Spectroscopy: Our Bruker Avance III HD 600 MHz NMR spectrometer facilitates non-destructive structural elucidation of flavanonol compounds, providing detailed insights into their chemical structure and conformation.
High-Resolution Mass Spectrometry (HR-MS): Leveraging instruments like the Thermo Scientific™ Orbitrap Exploris™ 240 Mass Spectrometer, we achieve superior mass accuracy and resolution for precise identification and characterization of flavanonols.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Flavanonols Analyzed (including but not limited to)
| Eriodictyol |
Taxifolin |
Pinobanksin |
Sakuranetin |
Naringenin |
| Hesperetin |
Fustin |
Isosakuranetin |
Homoeriodictyol |
Pinocembrin |
| Tricetin |
Chrysin |
Galangin |
Apigenin |
Eupatorin |
| Myricetin |
Kaempferol |
Morin |
Rhamnetin |
Quercetin |
| Fisetin |
Luteolin |
Diosmetin |
Tangeretin |
Luteolin-7-glucoside |
| Naringin |
Hesperidin |
Rutin |
Narirutin |
Poncirin |
Sample Requirements for Flavanonols Metabolism Assay
| Sample Type |
Sample Volume |
Sample Preparation |
| Plant Extracts |
≥ 100 μL |
Filtration and solvent extraction for efficient compound recovery |
| Fruit Juices |
≥ 200 μL |
Dilution with suitable solvents for concentration adjustment |
| Herbal Supplements |
≥ 100 mg |
Extraction using appropriate solvents and filtration |
| Biological Fluids |
≥ 200 μL |
Protein precipitation and centrifugation for clean sample preparation |
| Tissue Samples |
≥ 20 mg |
Homogenization in extraction buffer followed by centrifugation |
| Cell Culture Supernatant |
≥ 500 μL |
Precipitation of proteins or direct injection after filtration |
| Food Products |
≥ 2 g |
Homogenization and extraction using suitable solvents |
| Cosmetics |
≥ 100 mg |
Extraction with appropriate solvents and filtration |
| Environmental Samples |
Variable |
Filtration and solvent extraction based on sample matrix |
Advantages of Flavanonol Analysis at Creative Proteomics
Expertise: Our team comprises seasoned experts with extensive experience in flavanonol analysis, ensuring accurate results and insightful interpretations.
Customization: We tailor our analysis protocols to suit specific research objectives and sample characteristics, ensuring optimal outcomes.
Comprehensive Solutions: From identification to quantification and structural elucidation, we offer end-to-end solutions for flavanonol analysis.
Quality Assurance: Rigorous quality control measures are implemented at every step to ensure data integrity and reliability.
Deliverables of Flavanonols Analysis
- Comprehensive Report: Detailed report summarizing analysis methods, results, and interpretations.
- Spectral Data: Raw data files including LC-MS spectra, NMR spectra, and chromatograms.
- Structural Elucidation: Structural elucidation of identified flavanonols.
- Consultation: Expert consultation to discuss results and implications for further research or applications.
Case. Stilbene Determination in Wines: Optimized Liquid Chromatography with Fluorescence Detection
Background:
Stilbenes, bioactive compounds abundant in grapes and wine, have garnered attention for their potential health benefits, including cardiovascular protection and anticancer properties. However, the flavanonols, another class of phenolic compounds in wine, remain underexplored despite their potential contribution to wine's health properties.
Sample:
Forty-nine commercially available wines from AOC Bergerac, comprising blended red wines, dry white wines, and sweet white wines, were analyzed. This sample set represents wines from South-Western France, known for its diverse grape cultivars and winemaking traditions.
Technical Platform and Procedure:
The analysis of flavanonols, specifically astilbin and dihydromyricetin-3-O-rhamnoside, was conducted using a liquid chromatography (LC) system equipped with a UV absorbance detector. The chromatographic conditions closely resembled those used for stilbenes, with slight modifications to optimize separation and detection of flavanonols.
Sample Preparation:
- Wine samples were filtered to remove particulate matter and debris before analysis.
- A 100 μl aliquot of filtered wine sample was directly injected into the LC system without any prior sample treatment or extraction.
Chromatographic Conditions:
- A Prontosil C18 column (4.0 mm × 250 mm, 4 μm particle size) was utilized for separation.
- The mobile phase consisted of a gradient elution system comprising water/trifluoroacetic acid (TFA) (99/1, v/v) as solvent A and acetonitrile/solvent A (80/20, v/v) as solvent B.
- The flow rate was maintained at 1.0 ml/min throughout the analysis.
- The gradient program was optimized to achieve efficient separation of flavanonols and other compounds present in the wine matrix.
- Chromatograms were monitored at 290 nm using the UV absorbance detector, allowing for the detection and quantification of flavanonols based on their absorbance characteristics at this wavelength.
Calibration and Validation:
- Calibration curves for astilbin and dihydromyricetin-3-O-rhamnoside were constructed by injecting standard solutions with concentrations ranging from 0.1 to 10 mg/l.
- The calibration graphs were generated by plotting the concentration of each compound against its corresponding peak area, with each point representing the mean value from three independent area measurements.
- Regression coefficients, detection limits, quantification limits, and linearity intervals were determined for each compound to assess the method's sensitivity and accuracy.
Data Analysis:
- The concentrations of astilbin and dihydromyricetin-3-O-rhamnoside in wine samples were determined by comparing their peak areas with those of the standards and applying the calibration curves.
- Mean concentrations and standard deviations were calculated for each compound in red and white wine samples, providing insights into their abundance and variability across different wine types.
Results
The developed LC method enabled the quantification of astilbin and dihydromyricetin-3-O-rhamnoside in wines. Red wines exhibited higher levels of astilbin compared to white wines, with mean concentrations of 10 mg/l and 2.2 mg/l, respectively. Additionally, dihydromyricetin-3-O-rhamnoside levels were higher in both red and white wines, with mean concentrations of 44.7 mg/l and 3.0 mg/l, respectively. These findings highlight the presence of flavanonols in wines and their potential contribution to wine's health properties, warranting further investigation into their levels and health effects.
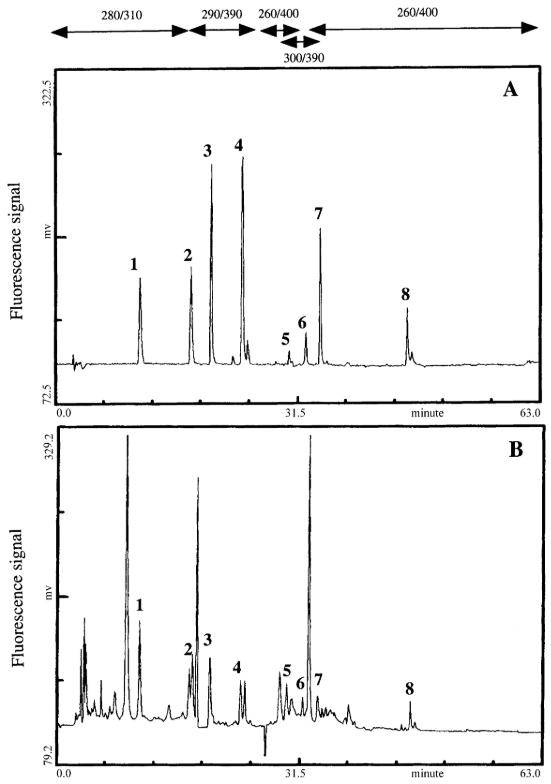 Chromatographic profiles using fluorescence detection for a standard mixture of polyphenols (A) and a directly injected sample of red wine (B)
Chromatographic profiles using fluorescence detection for a standard mixture of polyphenols (A) and a directly injected sample of red wine (B)
Reference
- Vitrac, Xavier, et al. "Direct liquid chromatographic analysis of resveratrol derivatives and flavanonols in wines with absorbance and fluorescence detection." Analytica Chimica Acta 458.1 (2002): 103-110.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Chromatographic profiles using fluorescence detection for a standard mixture of polyphenols (A) and a directly injected sample of red wine (B)
Chromatographic profiles using fluorescence detection for a standard mixture of polyphenols (A) and a directly injected sample of red wine (B)