Cancer metabolism is significantly altered from normal cellular metabolism. Reprogrammed cellular metabolism allows cancer cells to maintain high proliferation rates and adapt to changing microenvironments. Over the past decade, stable-isotope tracing and network analysis has become a powerful tool to reveal differentially activated metabolic pathways in cancer cells. Notably, 13C metabolic flux analysis (13C-MFA) has become an important technique for quantifying intracellular fluxes in cancer cells.
Overview of cancer metabolism
Cancer metabolism is a particularly active area of research, and it has long been recognized that cancer cells exhibit a rewired metabolism compared to normal cells. These early features of metabolic alterations suggest that cancer cells exclusively increase glycolytic flux to maintain high rates of ATP production. Thus, aerobic glycolysis, also known as the Warburg effect, is a major hallmark of cancer metabolism. More recently, additional metabolic pathways activated in cancer cells have been identified using stable isotope tracing and network analysis, such as altered glycolysis, reductive metabolism of glutamine, acetate and glycine metabolism, acetate metabolism, and one-carbon metabolism. The activity of these pathways allows cancer cells to use cellular components and energy from substrates for their growth. With rapid advances in cancer research, it is becoming increasingly clear how cancer cells rewire their metabolism to promote the proliferation of more cancer cells.
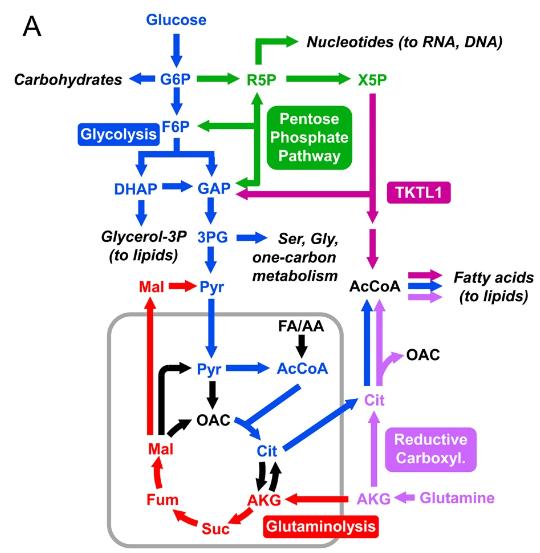 Fig. 1 The diagram shows important metabolic pathways in cancer metabolism. (Antoniewicz, Maciek R., 2018)
Fig. 1 The diagram shows important metabolic pathways in cancer metabolism. (Antoniewicz, Maciek R., 2018)
Our service
Although 13C-based metabolic flux analysis in cell culture studies has generated an amount of valuable data, only a few studies have used modeling approaches to fully explore the results obtained. To help researchers study the cellular metabolism of multiple cancer cells in-depth and comprehensively, we offer customized one-stop MFA analysis for cancer cells. In particular, we can combine MFA with metabolomics to quantify substrate flux through various metabolic pathways in cancer cells. The metabolic phenotype is highly heterogeneous across cancer types. We are focusing on several cancers, including glioblastoma, breast cancer, and pancreatic cancer.
To achieve high resolution of multiple metabolic pathways, we perform parallel labeling experiments with different tracers and then integrate all data into a single comprehensive flux model. Based on several GC/MS-based protocols, we can easily and accurately determine the biomass composition of cells. Our service can help investigators to study the following important metabolic pathways in cancer metabolism:
- Glycolysis
- Pentose phosphate pathway
- TCA cycle
- Reductive carboxylation of glutamine
- Transketolase-like 1 (TKTL1) pathway
Applications of our service
As a hallmark of cancer, metabolic reprogramming has been increasingly reported to be associated with tumorigenesis, progression, metastasis and drug resistance. MFA provides a suitable framework for understanding metabolic dysregulation, facilitating the discovery of therapeutic targets and the development of drugs for cancer treatment. Based on our advanced MFA analysis platform, we can help investigators to study the following lists:
- Uncover complex mechanisms of various cancers
- Uncover complex mechanisms of drug action and resistance
- Unravel novel drug targets
- Identify cancer biomarkers
- Contribute to precision treatment with pharmacometabolomics

References
- Antoniewicz, Maciek R. "A guide to 13C metabolic flux analysis for the cancer biologist." Experimental & molecular medicine 50.4 (2018): 1-13.
- Liang, Lingfan, et al. "Metabolomics, metabolic flux analysis and cancer pharmacology." Pharmacology & Therapeutics 224 (2021): 107827.