The growing demand for food and the increasing reliance on plant-derived drugs have increased the need to optimize plant metabolism. Despite the discovery of new enzymes and their functions, the specific pathways of cells and/or organelles, developmental stages and biotic/abiotic stress responses remain very unclear. Moreover, intracellular metabolic fluxes vary according to different cellular needs and physiological states, so metabolic fluxes cannot be generalized at any spatial or temporal scale. Therefore, additional information on metabolic fluxes is even more important than the metabolites themselves in order to obtain a true plant metabolic phenotype. Creative Proteomics is a leading provider of metabolic flux analysis services. Our team is dedicated to helping researchers and professionals accurately characterize complex metabolic pathways and phenotypes in plants.
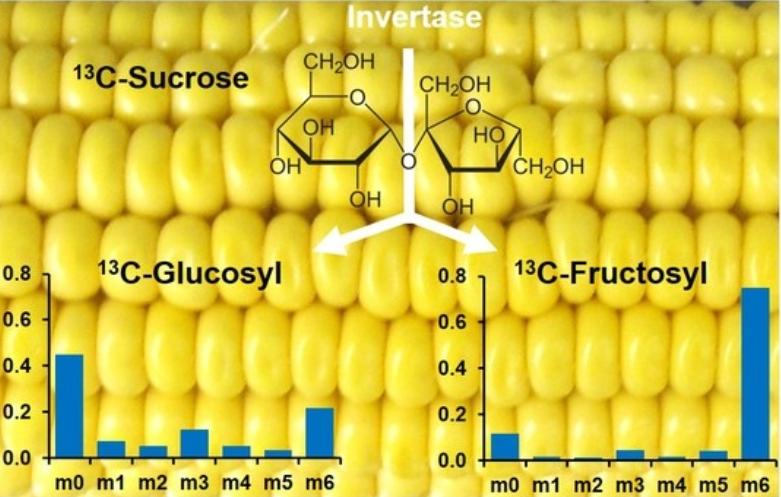 Fig. 1 Liquid chromatography tandem mass spectrometry quantification of 13C-labeling in sugars. (Cocuron, Jean-Christophe, Zacchary Ross, and Ana P. Alonso, 2020)
Fig. 1 Liquid chromatography tandem mass spectrometry quantification of 13C-labeling in sugars. (Cocuron, Jean-Christophe, Zacchary Ross, and Ana P. Alonso, 2020)
Challenges of MFA in plants
MFA in eukaryotes is more complicated than MFA in prokaryotes. Indeed, plants are highly compartmentalized at the tissue and subcellular levels. In addition to the high heterogeneity of cell types, plant cells have a number of subcellular compartments. Thus, different pools of the same metabolite may be present in different compartments, and some reactions and even entire pathways are duplicated, increasing the flexibility of metabolism. Another challenge of 13C-MFA in plants is to achieve a steady state at the isotopic and metabolic levels. To avoid significant metabolic shifts, the time factor required to reach both steady states has to be short, which may lead to a misconception of the 13C-MFA study.
Service offering at Creative Proteomics
Metabolic flux is the flow of substances through metabolic steps or pathways. The goal of 13C-MFA is to quantify all in vivo intracellular metabolic fluxes in a given organ or cell, resulting in a metabolic flux map. Except for some fluxes, such as substrate uptake rate and product accumulation, which can be measured directly, the determination of intermediate carbon fluxes is required for 13C-labeling. The labeled plant tissues/cells are usually harvested after they have reached an isotopic steady state, i.e., when the labeling in the intermediate metabolites and products has reached a constant pattern. We offer a variety of techniques to identify resultant labeling in a range of metabolites, including nuclear magnetic resonance (NMR) and mass spectrometry (such as GC-MS and LC-MS). Of these, NMR can provide positional isotope information and MS can give more accurate mass isotope information.
Our plant MFA services are not limited to tissues with long metabolic steady states and presumed homogeneous cell populations. In addition to cell suspensions, cultured roots, and developing seeds, we are able to determine other metabolically more dynamic tissues in greater detail based on advanced MS techniques, isotope labeling strategies, and transient labeling-based flux analysis, as well as improved computational modeling.
Our capabilities
- 13C metabolic flux analysis (13C-MFA)
- 15N metabolic flux analysis (15N-MFA)
- Non-target metabolic flux analysis
- Dynamic metabolic flux analysis (DMFA)
- Isotopic non-stationary 13C metabolic flux analysis (13C-NMFA)
- Flux balance analysis (FBA)
Applications of our service
- Uncovering the molecular mechanisms of plant stress resistance
- Evaluation of plant performance
- Assisted breeding
- Analysis of active ingredients
Our precise, customized, and flexible services provide a more realistic picture of plant function and offer insights into target genes that need to be modified as natural resources become increasingly limited and the environment changes. If you are interested in MFA in plants, please contact us. Our professional experts will provide you with further knowledge or consulting services.
References
- Shih, Meng-Ling, and John A. Morgan. "Metabolic flux analysis of secondary metabolism in plants." Metabolic engineering communications 10 (2020): e00123.
- Koley, Somnath, Manish L. Raorane, and Björn H. Junker. "Shoot tip culture: a step towards 13C metabolite flux analysis of sink leaf metabolism." Plant methods 15.1 (2019): 1-15.
- Cocuron, Jean-Christophe, Zacchary Ross, and Ana P. Alonso. "Liquid chromatography tandem mass spectrometry quantification of 13C-labeling in sugars." Metabolites 10.1 (2020): 30.