Protein-Protein Interaction Analysis Service
As functional molecules in cells, proteins are responsible for most life activities, but it requires interacting with other proteins. Therefore, protein-protein interaction (PPI) is essential for proteomics research. With years of experience in proteomics research, Creative Proteomics can provide professional protein-protein interaction analysis services to facilitate your protein research.
What is Protein-Protein Interaction
PPI means the bonding process between two or more proteins, usually intended to perform their biological functions which are realized usually relying on interactions and chemical reactions between biological molecules. Proteins need to coordinate with each other to collectively achieve complex cellular functions in general, whereas a single protein is difficult to function. PPIs are ubiquitous in life activities and indispensable in biochemistry, metabolism, genetics, molecular biology, and other research fields. In fact, in all living cells, PPIs are at the center of various interactions and present in almost all biochemical reactions. By researching PPIs, people can gain a deeper understanding of disease development and open up new ideas for developing new therapies.
The Principle of Protein-Protein Interaction Analysis
The study of PPIs can help us better understand cells' life activities and reveal regulatory mechanisms under the surface. And the current main technical methods to research PPI include yeast two-hybrid, Co-IP, fluorescence colocalization, GST-pull down, and BiFC, etc. By learning the principles and characteristics of various research methods, we can choose the appropriate methods according to different experiment requirements and purposes.
Yeast two-hybrid is using two domains required for gene transcription to bind to two proteins respectively. If there is an interaction between these two proteins, the domains will bind together to induce reporter gene expression. By detecting whether a target gene product is formed, it determines whether there is an interaction between the two proteins to be tested.
Co-IP uses the specific binding between antibodies and target proteins to pull down proteins that can interact with the target protein in the sample. Then, the obtained proteins were analyzed using SDS-PAGE, Western Blot, mass spectrometry, etc., to prove the PPI.
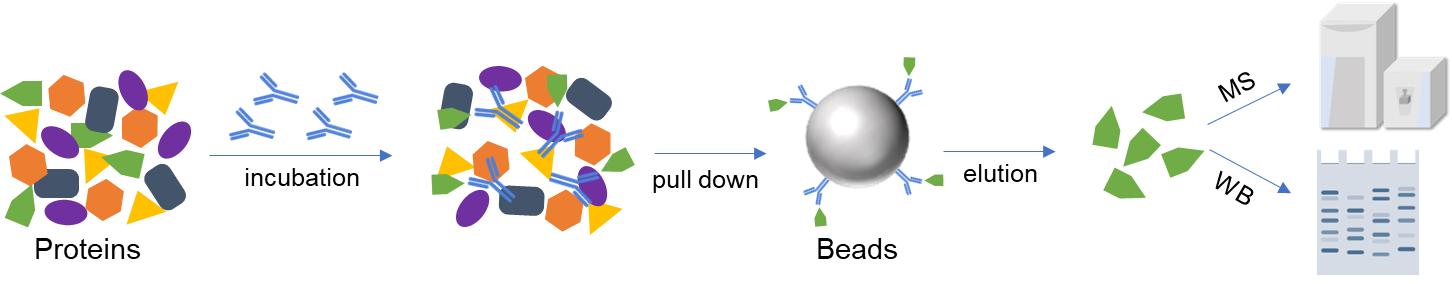
Fig. 1. Schematic of Co-IP
Fluorescence colocalization combines immunological methods with fluorescent labeling techniques to analyze PPIs. Using different fluoresceins to label the two proteins, and then, observing whether there is colocalization of frequencies can verify whether there is an interaction between the two proteins.
GST pull down requires constructing a target protein-GST vector first and immobilizing it on a glutathione affinity resin. The sample is then passed through the resin, which captures the interacting protein. Next, the target proteins were eluted, and the protein interactions were verified by SDS-PAGE, western blot, etc.
BiFC first cuts the fluorescent protein into two segments at specific sites and binds them to two proteins separately. If two proteins could interact with each other, the two fragments would be reconstituted into a complete fluorescent protein, which can be excited to produce fluorescence. Under a fluorescence microscope, it will be directly observed whether the two target proteins interact or not.
Although there are many ways to study PPI, choosing the appropriate analytical method for your research requirements is the optimal solution. The following is an overview of each method's advantages and disadvantages.
Table 1. Advantages and disadvantages of each method
| Methods | Advantages | Disadvantages |
| Yeast two-hybrid | High-throughput screening Reacts to intracellular reality Detects weak and transient interactions |
Many false positives and false negatives Difficult to judge the strength of the interaction Requires other methods to confirm results |
| Co-IP | Close to natural state Low false positive rate Quantifiable Proteins can be isolated |
Requires selection of specific antibodies Difficult to detect weak and transient interactions |
| Fluorescence colocalization | High specificity High sensitivity |
Problems with specific staining Lack of objectivity |
| GST pull down | Simple and flexible High throughput Quantifiable Direct detection |
Requires protein expression and purification May be false positive |
| BiFC | In living cells Direct observation |
May be false positive Requires negative control |
Our Service
Creative Proteomics provides considerate and high-quality protein-RNA interactions analysis services. And we offer all the analytical methods described above and can provide you with the optimal solution for your research requirements. In addition, we provide protein-metabolite interaction analysis service, protein-RNA interaction analysis service and protein-DNA interaction analysis service.
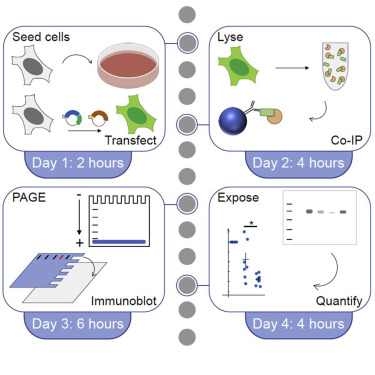
Fig. 2. Co-IP workflow (Burckhardt C J, et al., 2021)
Applications of Protein-Protein Interaction Analysis
Protein-protein interactions are widespread in a variety of biological activities, and the study will help to reveal life processes, such as intercellular information transfer, material transport, biochemical reactions, etc., and it can provide a theoretical basis for disease research, medical research, drug research and other fields.
Advantages of Our Service
- Multiple methods are available.
- Research from multiple perspectives.
- Multiple methods can be used in combination.
Creative Proteomics provides comprehensive and high-quality proteomics analysis services. If you are interested in protein-protein interaction analysis, please contact us for more details.
Reference
- Burckhardt C J.; et al. Co-immunoprecipitation and semi-quantitative immunoblotting for the analysis of protein-protein interactions. STAR Protocols. 2021, 2(3): 100644

