Functional Protein Microarray Service
What is a Functional Protein Microarray?
A functional protein microarray is a high-density platform that displays correctly folded and biologically active proteins immobilized onto a solid surface. Unlike peptide arrays or denatured protein arrays, functional arrays preserve the native conformation of proteins, enabling the study of physiologically relevant binding interactions.
The protein microarray allows thousands of proteins to be interrogated simultaneously using minimal sample volume. This enables researchers to evaluate autoantibody responses, protein–protein interactions, enzyme activities, or small molecule binding in a single experiment. The ability to study native-fold proteins ensures more accurate representation of biological events, making functional protein microarrays a powerful tool in both discovery research and applied drug development.
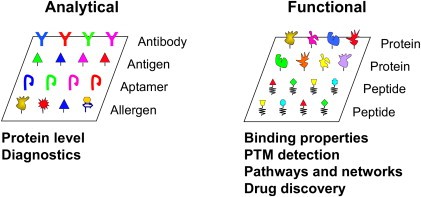
Figure 1. Classification of protein microarrays: analytical and functional protein microarrays (Zhu H, Qian J., 2012).
Why Choose Functional Protein Microarrays Over Other Proteomic Screens?
Functional protein microarrays offer several advantages over conventional proteomics and immunoassays:
- Native structure preservation: folding markers and optimized immobilization ensure biologically active proteins.
- High throughput: thousands of targets screened simultaneously, accelerating biomarker discovery.
- Sample efficiency: requires only microliter amounts of serum, plasma, or other biofluids.
- Functional readouts: capture interactions with antibodies, ligands, or small molecules in a physiologically relevant context.
Functional protein microarrays combine scale, sensitivity, and versatility compared to methods such as ELISA, Western blotting, or Co-Immunoprecipitation. They complement mass spectrometry-based proteomics by providing an accessible and reproducible screening format for hypothesis generation.
Creative Proteomics' Functional Protein Microarray Service Workflow
Our functional protein microarray service follows a robust and validated workflow to ensure reproducible results:
- Protein Selection and Array Design: Native proteins, domains, or panels of interest are selected and immobilized.
- Protein Expression and Folding QC: Proteins are expressed with folding markers and validated for correct structure.
- Microarray Printing: High-density spotting on hydrogel or glass substrates preserves function.
- Detection: Bound antibodies or ligands are detected using labeled secondary antibodies (IgG, IgA, IgM) or label-free methods.
- Data Acquisition: Arrays are scanned for fluorescent or chemiluminescent signals.
- Bioinformatics Analysis: Data normalization, statistical modeling, clustering, and pathway enrichment.

Protein Folding, Quality Control, and Functional Integrity
Only correctly folded proteins are biotinylated and immobilized, ensuring that immobilized proteins retain biological activity. Our platform is the use of biotin carboxyl carrier protein (BCCP) fusion tags as folding markers. Additional QC measures include:
SDS-PAGE and Western blot validation
Functional ligand-binding assays
Internal positive/negative controls printed on each array
Replicates for statistical reproducibility
Deliverables and Reporting Standards
- Raw Data Files: Fluorescence or signal intensity values for each protein feature, including replicate spots and control signals.
- Normalized Data: Background-subtracted, scaled, and normalized values suitable for cross-sample comparison.
- Hit Lists: Proteins or targets showing statistically significant interactions or binding events, annotated with fold-change, p-values, and confidence scores.
- Visualizations: Heatmaps, volcano plots, and cluster diagrams to quickly identify patterns or trends.
- QC Reports: Signal-to-noise ratios, intra- and inter-array variability, positive/negative control performance.
- Interpretation Notes: Brief summary of key findings, recommended follow-up experiments, and biological relevance.
Applications of Functional Protein Microarray
- Autoantibody Biomarker Discovery: Profiling immune signatures for early disease diagnostics, including cancer, autoimmune, and infectious diseases.
- Cancer Neoantigen Identification: Detecting tumor-associated antigens and immune recognition targets for personalized immunotherapy development.
- Drug and Biologic Selectivity Screening: Assessing specificity and off-target effects of monoclonal antibodies, biologics, and small molecules.
- Adverse Drug Reaction Prediction: Identifying autoantibody biomarkers associated with immunogenicity or drug hypersensitivity.
- Protein-Protein Interaction and Functional Studies: Screening novel interactions between signaling proteins, transcription factors, and extracellular proteins.
- Vaccine Development: Mapping antigen–antibody interactions to evaluate vaccine candidates and immune responses.
Sample Requirements
| Sample Type | Recommended Volume | Storage Conditions | Notes |
| Serum | 20–50 µL | -80°C, avoid repeated freeze-thaw | Use clot activator tubes; centrifuge to remove cells |
| Plasma | 20–50 µL | -80°C, avoid repeated freeze-thaw | Collect in EDTA, citrate, or heparin tubes; remove platelets |
| CSF | 10–30 µL | -80°C | Avoid contamination with blood; centrifuge if necessary |
| Other body fluids | 20–50 µL | -80°C | Pre-clarify by centrifugation; consult for unusual fluids |
| Hemolyzed samples | Not recommended | N/A | Hemoglobin may interfere with detection |
| Lipemic samples | Not recommended | N/A | High lipid content may affect signal intensity |
Why Choose Creative Proteomics for Functional Protein Microarray Service
- Extensive Expertise: Rich experience in proteomics, biomarker discovery, and functional protein analysis.
- High-Quality Protein Panels: Proteins are correctly folded, functionally validated, and quality-controlled to preserve native activity.
- Customizable Arrays: Tailored panels for disease-specific research, drug selectivity screening, or autoantibody profiling.
- Comprehensive Data Analysis: Bioinformatics support for hit identification, pathway analysis, and integration with complementary proteomics approaches.
- Flexible Service Models: Options for academic research, CRO projects, and pharma R&D, including discovery and validation workflows.
- Client-Centric Project Management: Dedicated technical consultation, secure data handling, and clear communication throughout the project.
FAQ
-
Q1: Can multiple antibody types be detected simultaneously?
A1: Yes. We can profile IgG, IgA, IgM, and other antibody types at the same time. This allows a comprehensive view of the immune response in a single experiment.
-
Q2: Can the protein arrays be customized for specific research needs?
A2: Absolutely. We offer custom panels tailored to your project, including disease-specific proteins, signaling molecules, or any proteins of interest. Panels can be designed for discovery or targeted validation studies.
-
Q3: Are there any limitations should be aware of?
A3: Functional protein microarrays work best for soluble proteins and antibodies. Very low-abundance targets may require enrichment or orthogonal validation. We recommend complementary follow-up methods such as mass spectrometry or Co-IP for confirmation.
-
Q4: Can the assay distinguish between closely related proteins?
A4: Yes. Arrays are designed with carefully validated proteins to minimize cross-reactivity, allowing distinction between isoforms or homologous proteins in most cases.
Demo
Demo: A quantitative protein interaction network for the ErbB receptors using protein microarrays
This study employed protein microarrays containing nearly all human SH2 and PTB domains to map interactions with 61 tyrosine-phosphorylated peptides from the four ErbB receptors, generating over 77,000 measurements. The resulting quantitative network revealed 116 novel interactions and showed distinct receptor behaviors: ErbB2 exhibited high promiscuity, EGFR and ErbB2 became more promiscuous at higher concentrations, while ErbB3 remained selective. These findings highlight differences in receptor signaling dynamics and suggest that concentration-dependent promiscuity may underlie the strong oncogenic potential of EGFR and ErbB2, offering insights for therapeutic strategies.
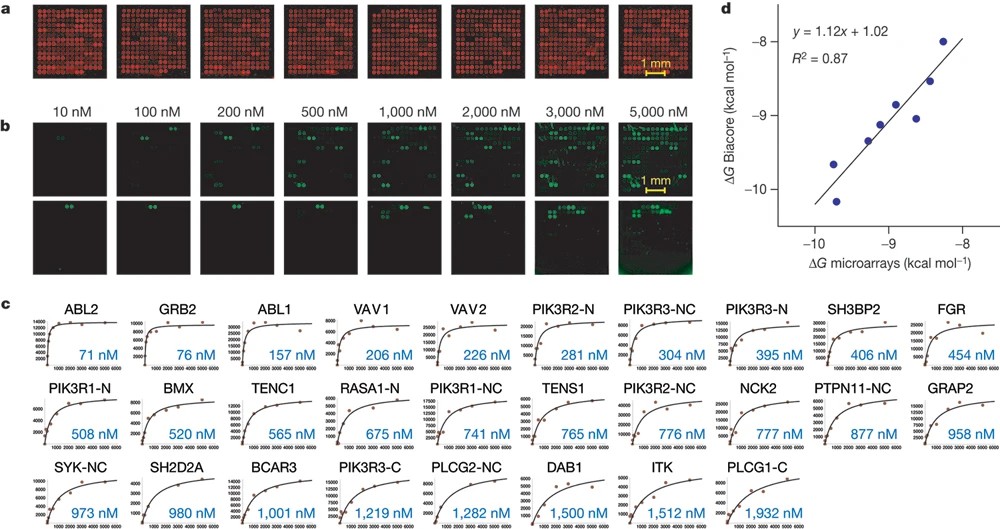
Figure 2. Measuring the binding affinity of SH2/PTB domains for phosphopeptides derived from the ErbB receptors using protein microarrays (Jones R B, et al., 2006).
Demo: SARS-CoV-2 multi-antigen protein microarray for detailed characterization of antibody responses in COVID-19 patients
The group constructed a protein microarray, then profiled patient sera to map binding across variants in parallel. The microarray enabled simultaneous binding and variant-specific analyses from microliter samples and helped distinguish infection vs vaccine-elicited responses and variant cross-reactivity. Methodologically it is a clear example of applying functional/antigen microarrays for high-throughput immunomonitoring.
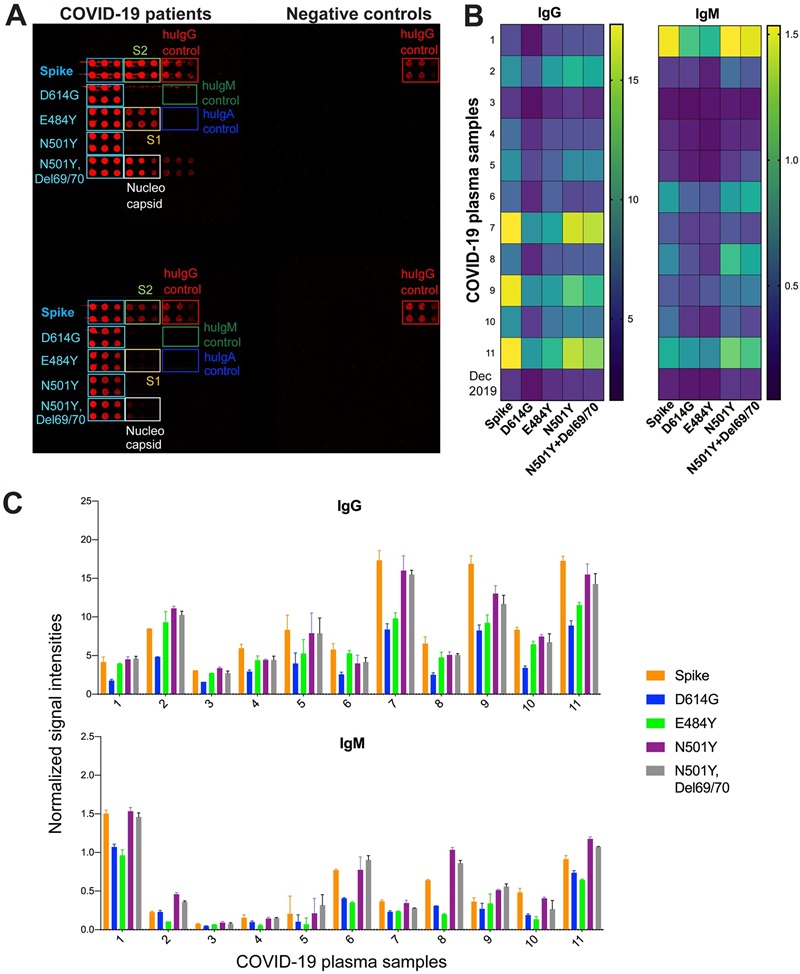
Figure 3. Antibody responses to S variants with single and double mutations in sera from COVID-19 patients (Celikgil A, et al., 2023).
-
Case Study
Case: Identification of potential autoantigens in anti-CCP-positive and anti-CCP-negative rheumatoid arthritis using citrulline-specific protein arrays.
Abstract
Antibodies against citrullinated proteins (ACPAs) are a hallmark of many rheumatoid arthritis (RA) patients and define clinically and pathogenically distinct patient subgroups. Mapping the breadth of citrullinated protein targets (the "auto-antigenome") can reveal candidate biomarkers and provide insight into PAD enzyme (PAD2/PAD4) contributions to neoepitope generation. To identify proteins that become recognized by ACPAs after in-array citrullination and to compare PAD2 versus PAD4 efficiency in generating ACPA-binding citrullinated epitopes in plasma from anti-CCP-positive and anti-CCP-negative RA patients. The study aimed to generate a large candidate list of potential citrullinated autoantigens for downstream validation.
Methods
- Platform: A high-density protein microarray containing 1,631 full-length proteins printed in quadruplicate was used.
- Samples: Plasma pools from 15 anti-CCP-positive and 10 anti-CCP-negative RA patients (pooled separately) were applied.
- PTM treatment: Arrays were treated with recombinant human PAD2 or PAD4 to generate citrullinated proteins under defined buffer conditions.
- Detection & analysis: Bound IgG autoantibodies were detected by fluorescent scanning; raw intensities underwent background subtraction, quantile/intensity normalization, z-score filtering and statistical testing.
Results
- After citrullination, 844/1,631 arrayed proteins were recognized by ACPAs under the assay conditions, indicating extensive ACPA reactivity across many proteins.
- Applying additional filtering to prioritize higher-intensity and reproducible signals yielded ≈100 high-confidence potential autoantigens.
- PAD2 and PAD4 showed broadly similar efficiency in generating epitopes recognized by ACPAs at the tested serum dilution; only a handful of proteins displayed PAD-isoform-preferential ACPA binding.
- The authors note that many hits were low-intensity and emphasize that in-vitro citrullination does not guarantee in-vivo relevance without orthogonal validation.
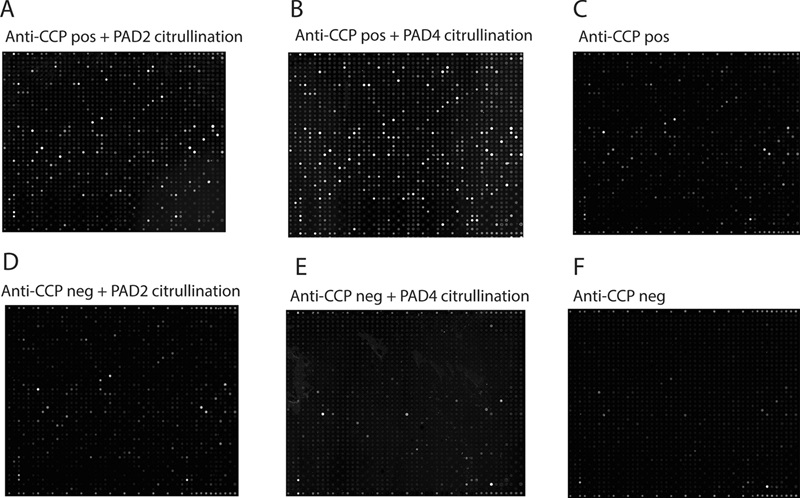
Figure 4. Imaging of autoantibody binding to citrullinated and non-citrullinated protein arrays.
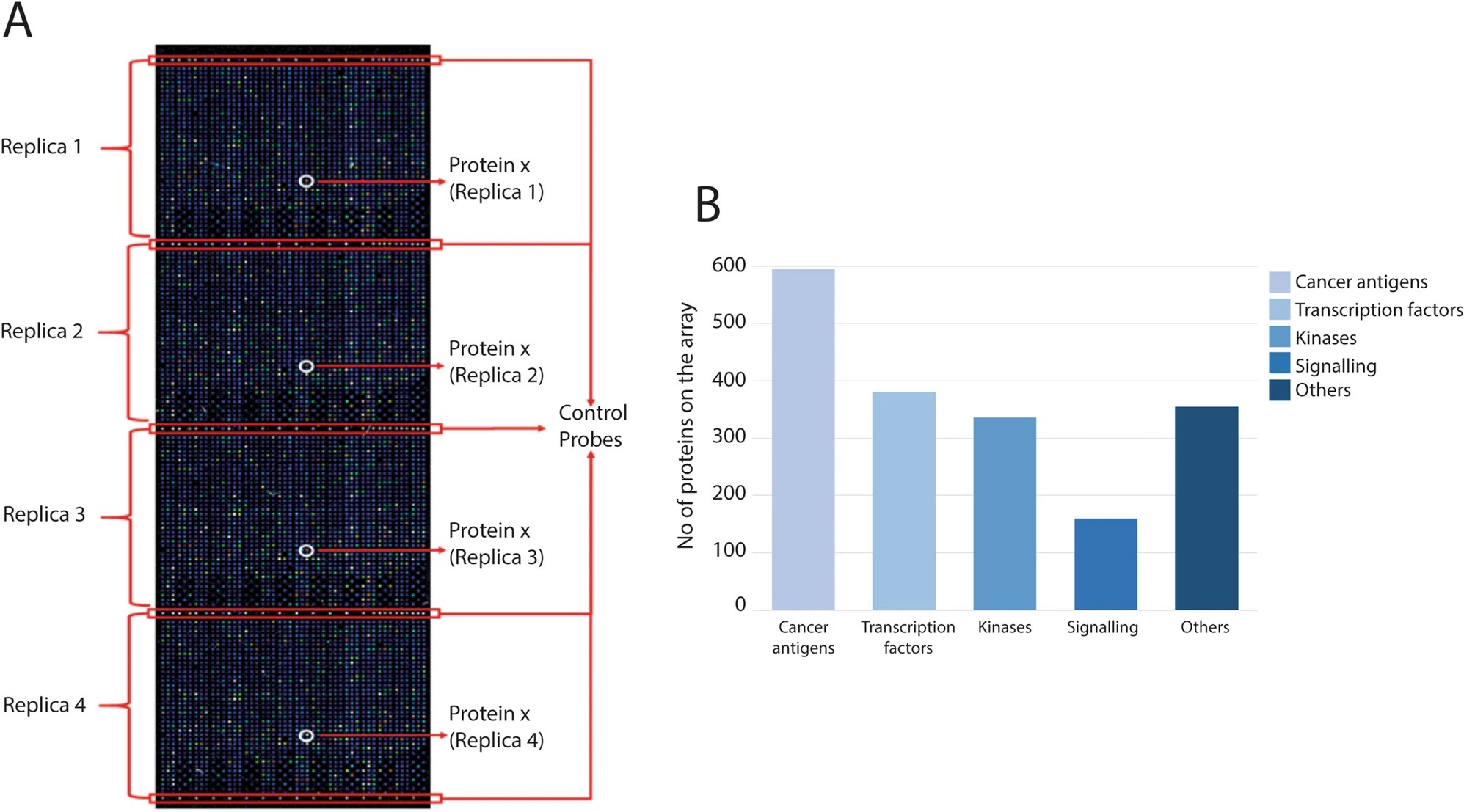
Figure 5. Protein categories on the protein microarray.
Conclusion
The study demonstrates that high-density functional protein microarrays provide a scalable approach to map candidate citrullinated autoantigens in RA. The large set of ACPA-reactive proteins represents a resource for hypothesis generation and subsequent validation by orthogonal methods. The comparable activity of PAD2 and PAD4 under the experimental conditions suggests both enzymes can generate ACPA-recognized neoepitopes, although biological relevance requires further investigation.
Related Services
References
- Zhu H, Qian J. Applications of functional protein microarrays in basic and clinical research. Advances in genetics, 2012, 79: 123-155.
- Jones R B, et al. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature, 2006, 439(7073): 168-174.
- Celikgil A, et al. SARS-CoV-2 multi-antigen protein microarray for detailed characterization of antibody responses in COVID-19 patients. PLoS One, 2023, 18(2): e0276829.
- Poulsen T B G, et al. Identification of potential autoantigens in anti-CCP-positive and anti-CCP-negative rheumatoid arthritis using citrulline-specific protein arrays. Scientific Reports, 2021, 11(1): 17300.