- Services
- Demo
- Case Study
- FAQ
- Related Services
- Support Documents
- Inquiry
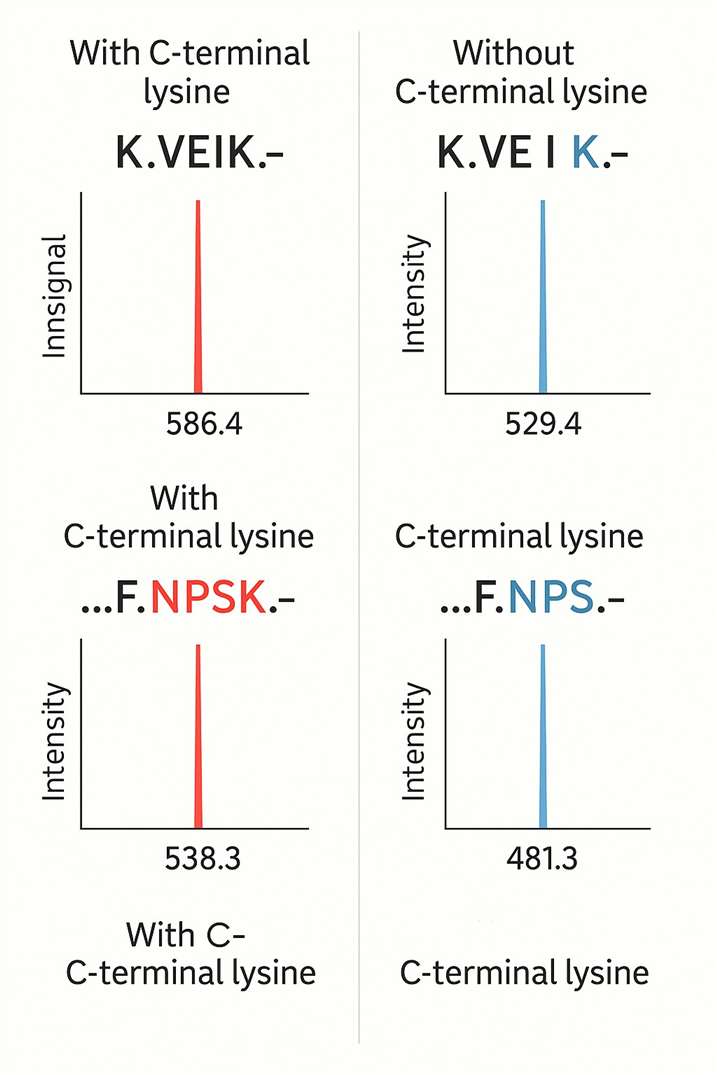
What is C-terminal lysine variant?
Monoclonal antibody (mAb) therapies are transforming modern medicine—but their complex structure often leads to molecular variation. One of the most common sources of this heterogeneity is the presence or absence of lysine residues at the C-terminal end of the antibody's heavy chain. While these lysine variants typically don't affect therapeutic function or structural integrity, they are a critical quality marker for product consistency during production and storage.
Why Measure C-Terminal Lysine Variants?
Even subtle molecular shifts can impact regulatory compliance and batch-to-batch reproducibility. Identifying and quantifying lysine variants supports:
- Regulatory submissions and lot release testing
- Fine-tuning of upstream and downstream processing
- Risk mitigation during formulation and storage
How to Determine The C-Terminal Lysine Variants?
Analytical Approaches: Why LC-MS Leads the Pack
Several analytical tools exist to assess C-terminal lysine heterogeneity:
- Isoelectric Focusing (IEF): Detects pI shifts caused by the basic nature of lysine.
- Ion-Exchange Chromatography (IEC): Separates variants by charge differences.
- LC-MS (Liquid Chromatography–Mass Spectrometry): Offers direct, high-resolution identification and quantification.
While IEF and IEC are useful for profiling charge variants, they fall short on specificity. In contrast, LC-MS allows precise identification of lysine-containing peptides—even at low abundance.
Our Workflow: From Protein to Precise Variant Identification
At Creative Proteomics, we've developed a sensitive and accurate LC-MS-based workflow:
- Enzymatic Digestion: Monoclonal antibodies are digested with trypsin to generate peptide fragments.
- Peptide Separation: Using a reversed-phase HPLC system and a C18 column, peptides are separated based on hydrophobicity.
- Mass Spectrometry Detection: We use tandem mass spectrometry (MS/MS) to detect unique ions corresponding to the C-terminal peptides.
- Data Interpretation: Single-charged ions are extracted and analysed for variant identification and quantification.
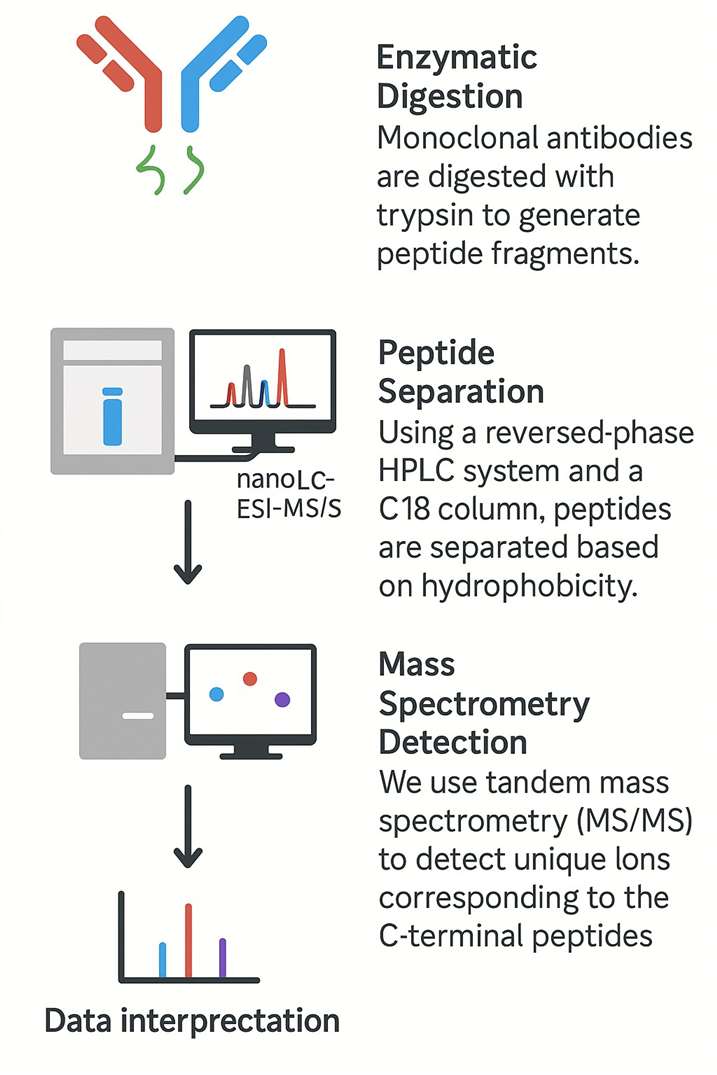
Our platform employs nanoLC-ESI-MS/MS technology, delivering high mass accuracy and sensitivity—ideal for both routine quality checks and investigative batch analysis.
Expert Insight at Every Step
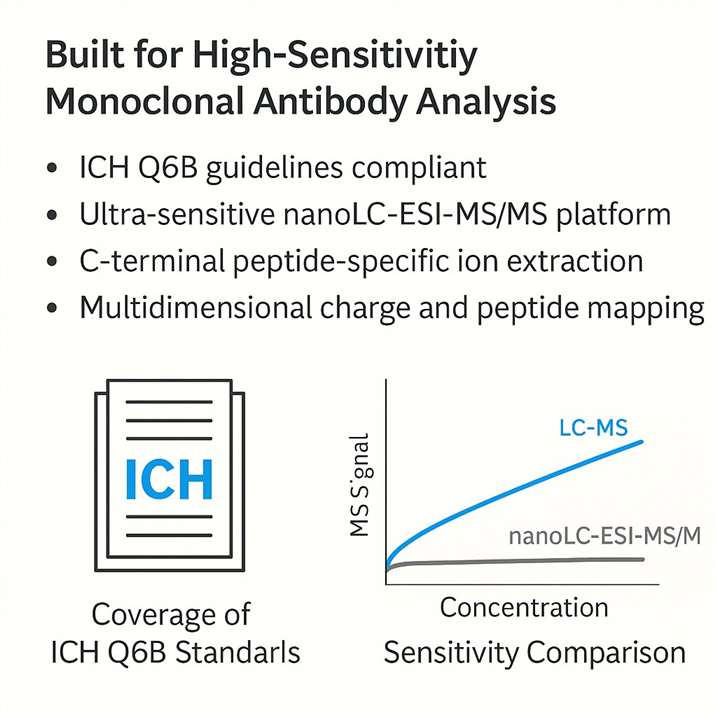
Platform Advantages for Monoclonal Antibody Consistency Analysis
- Regulatory-Ready Quality Control, Aligned with ICH Q6B
Our platform is purpose-built to support the comprehensive quality evaluation of monoclonal antibodies. Designed in full compliance with the ICH Q6B guidelines on specifications for biotechnological products, our workflows cover structural characterization, heterogeneity profiling, and quantitative benchmarks.
- Ultra-Sensitive nanoLC-ESI-MS/MS for Trace-Level Detection
Leveraging a high-resolution nanoLC-ESI-MS/MS system, we can detect C-terminal lysine variants at sub-micromolar levels—even within complex biological matrices. This level of precision enables early identification of product inconsistencies, empowering teams to proactively address potential quality issues.
- Proprietary Peptide Ion Extraction for Enhanced Specificity
Our in-house ion-filtering algorithm allows for rapid and accurate identification of C-terminal-specific peptide fragments and their modifications from large-scale MS datasets. This boosts both analytical speed and confidence, making it ideal for high-throughput batch analysis in antibody drug development.
- Integrated Charge Variant & Peptide Mapping Analysis
We combine charge variant profiling (via IEF and IEC) with LC-MS-based peptide mapping to deliver multidimensional insights. Our reports go beyond identifying the presence of variants—they also help trace their root causes, providing actionable data to refine manufacturing processes.
- Full Workflow Compatibility
From cell line screening to purification and formulation optimization, our platform supports detailed variant analysis at every critical step. This ensures a stable, controllable production process.
- Transparent, Traceable Data for Global CRO/CDMO Collaboration
We deliver complete raw data packages, annotated spectra, and standardized QC documentation.
Sample Submission Requirements
| Parameter | Requirement |
|---|---|
| Sample Type | Purified monoclonal antibody (mAb) or Fc-fusion protein |
| Purity | ≥ 90% (as confirmed by SDS-PAGE or SEC-HPLC) |
| Concentration | ≥ 1.0 mg/mL |
| Volume Required | ≥ 100 μL |
| Buffer Conditions | Preferably in PBS or formulation buffer without high salt or detergent |
| Avoid | Samples containing EDTA, DTT, Triton X-100, or high concentrations of surfactants |
| Storage & Shipping | Ship on dry ice or cold packs; store at –80°C for long-term |
| Additional Info | Provide antibody sequence (especially heavy chain C-terminal region) if available |
Demo Sample
Case Study: LC–MS-Based Analysis of C-Terminal Lysine Variants in Bevacizumab
LC–MS based case-by-case analysis of the impact of acidic and basic charge variants of bevacizumab on stability and biological activity.
Scientific Reports
https://www.nature.com/articles/s41598-020-79541-2
Background
Bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor (VEGF), exhibits charge heterogeneity due to post-translational modifications, notably C-terminal lysine variants. Understanding these variants is crucial for ensuring product consistency and efficacy.
Methodology
- Isolation of Charge Variants:
Semi-preparative cation exchange chromatography was employed to separate acidic and basic charge variants of bevacizumab. - Characterization:
- Intact Mass Analysis: Confirmed the molecular weights of the variants.
- Tryptic Peptide Mapping via LC–MS/MS: Identified the presence or absence of C-terminal lysine residues in the heavy chain.
- Stability and Activity Assessment:
Evaluated the structural integrity and biological activity of each variant.
Findings
- C-Terminal Lysine Variants:
Basic variants were attributed to incomplete removal of C-terminal lysine residues. These variants showed structural and functional profiles comparable to the main bevacizumab product. - Acidic Variants:
Resulted from deamidation and oxidation. One acidic variant exhibited a ~62% decrease in biological activity, highlighting the potential impact of certain modifications.
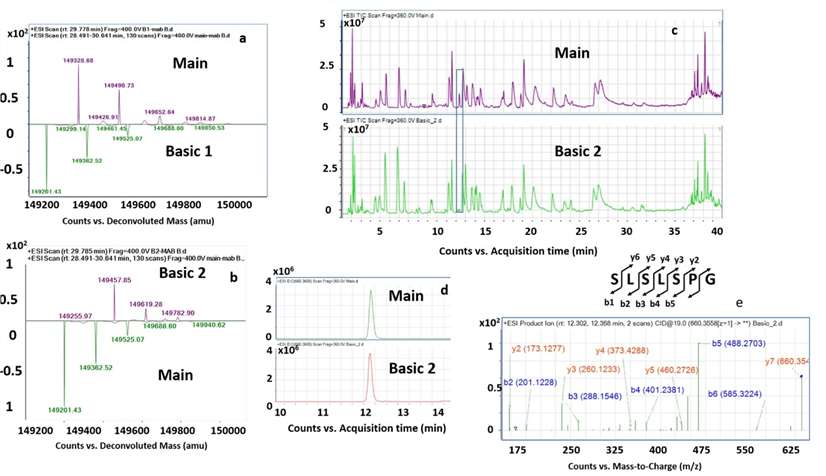 Figure 3 from the referenced study illustrates the separation and identification of bevacizumab charge variants using LC–MS/MS.
Figure 3 from the referenced study illustrates the separation and identification of bevacizumab charge variants using LC–MS/MS.Conclusion
LC–MS/MS is a powerful tool for characterizing C-terminal lysine variants in monoclonal antibodies. While basic variants may not significantly affect function, certain acidic modifications can impair biological activity, underscoring the importance of thorough analytical assessment.
References
- Iranian Biomedical Journal. http://ibj.pasteur.ac.ir/article-1-3837-en.html
- Conić, I., Nedović, B., Živadinović, R., Živadinović, R., Petrić, A., Stojnev, S., Petković, I., Krtinić, D., & Radić, M. (2024). Analysis of progression-free and overall survival in ovarian cancer: Bevacizumab treatment outcomes using historical cohort. PubMed. https://doi.org/10.1691/ph.2024.4570
- Annibale, B., Ribaldi, S., Anania, M., Stagnitti, F., Ström, R., Corleto, V., & Fave, G. D. (1989). Somatostatin Infused during Acute Pancreatitis Retains Its Biological Activity. Pancreas. https://doi.org/10.1097/00006676-198912000-00004
- Yan, T., Pan, Y., Yu, M. L., Hu, K., Cao, K. Y., & Jiang, Z. (2020). Full-Range Profiling of tRNA Modifications Using LC–MS/MS at Single-Base Resolution through a Site-Specific Cleavage Strategy. Analytical Chemistry. https://doi.org/10.1021/acs.analchem.0c03307
Frequently Asked Questions (FAQ)
Q: Can the analysis distinguish between single lysine truncation and complete deletion?
A: Yes. Our LC-MS/MS platform offers peptide-level resolution, allowing precise differentiation between peptides with one, two, or no C-terminal lysine residues.
Q: What information is included in the final report?
A: The report includes annotated MS/MS spectra of C-terminal peptides, sequence information, relative abundance percentages, variant site analysis, method conditions, and QC metrics. Both PDF reports and raw data files are provided.
Q: Is it possible to quantify the proportion of lysine variants?
A: Absolutely. We provide detailed quantification of each variant form, reporting the percentage abundance of lysine-modified versus unmodified peptides, supporting comparability and trend analysis.
Q: Is this service compatible with bispecific antibodies or Fc-fusion proteins?
A: es. Our workflow supports complex biologics, including bispecific antibodies and Fc-fusion formats. Simply provide your sequence, and we will tailor the enzymatic digestion and ion extraction accordingly.
Q: Can method validation or repeatability tests be provided?
A: Yes. For regulatory or QA purposes, we offer intra-batch repeatability assessments (e.g., %RSD), reference standard comparisons, and multi-batch consistency studies.
Q: Can C-terminal lysine analysis be combined with other PTM analysis?
A: Definitely. We can integrate C-terminal lysine analysis with monitoring of glycosylation, oxidation, disulfide mapping, or other post-translational modifications using a unified LC-MS platform.
Related Services
Support Documents
KNOWLEDGE CENTER











