- Services
- FAQ
- Case Study
- Related Services
- Support Documents
- Inquiry
What is Capillary Isoelectric Focusing (cIEF)?
Isoelectric point (pI) is the pH value at which the overall net charge of a macromolecule equals zero. pI is one of important physico-chemical parameters to influence overall pharmacokinetic behavior, so that it's necessary to assess pI during drug development.
pI can be determined by various experiments, including isoelectric focusing experiments, free flow electrophoresis, capillary electrophoresis, and in-gel electrophoresis experiments using immobilized pH gradient strips. Amphoteric compounds are fractionated depending on their pI along a continuous pH gradient in isoelectric focusing (IEF), which is the most common used electrophoretic technique to determine pI. And capillary IEF (cIEF) is the technique that can quantitatively and experimentally analyze the pI, and is widely adopted in most chemistry laboratories. Two ways to perform cIEF: coated or uncoated capillaries. In terms of using coated capillary, a two-step protocol is required due to the suppression of electroendoosotic flow, including a focusing step, and mobilization. As for uncoated cIEF, focusing happens when the entire pH gradient and train of bands is migrating to the capillary outlet. Since the coated cIEF has higher reproducibility and reduced chance of accidental protein absorption to the silica wall, it is more preferable and commonly used.
Determination of pI with precision is not an easy task, since a variety of experimental parameters affect the value, such as temperature, salt, solubilizers, calibration, etc. Our experienced scientific team will offer sensitive, accurate and reliable pI determination with cIEF for different types of samples.terization.
Methods of Isoelectric Point Determination with cIEF
In cIEF, pI markers are the only way for charting the course of pH gradients. Hence, employment of precise isoelectric point makers for cIEF is also critical. A set of 16 synthetic oligopeptides (trimers to hexamers), which have sharp focusing, stability, high purity and high solubility, will be used as isoelectric point makers to determine pI value. Each peptide in this set is made to contain one Trp residue for UV absorption detection and other residues with ionic side-chains for sharply-focusing peak during cIEF.
The samples will be first mixed with ampholytes, stablilizers and pI markers, and loaded to the capillary. These components are loaded into the capillary, which is then connected to the electrolyte reservoirs (anolyte and catholyte).
As the electric field is applied, hydronium (H₃O⁺) and hydroxide (OH⁻) ions from the respective electrolyte reservoirs create a pH gradient across the capillary. Proteins move along the gradient until they reach their pI, at which point they lose their net charge and become focused at a specific location. The pI value of the protein is determined based on its position within the gradient, which is tracked using UV absorbance or native fluorescence detection.
Experts from Creative Proteomics will optimize protocols and pI markers to provide precise pI determination of different types of samples, and customized services will be provided to help customers solve analytical and technical problems.
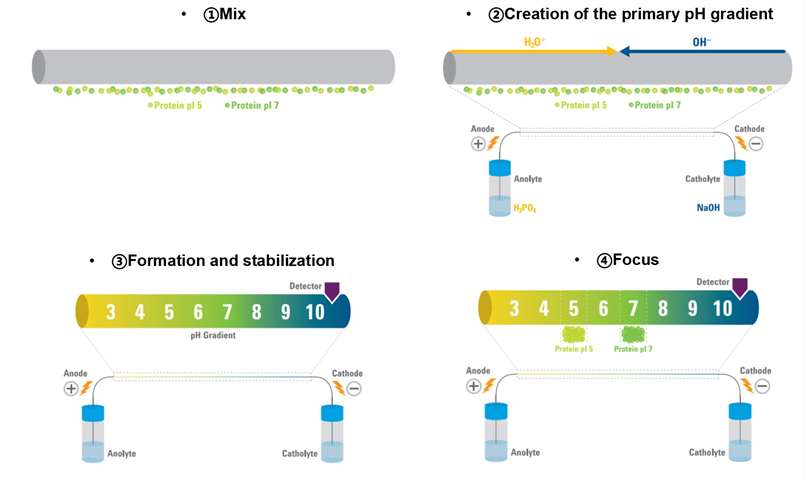 Figure 1. Steps of isoelectric point determination with cIEF (Kristl T, et al., 2014)
Figure 1. Steps of isoelectric point determination with cIEF (Kristl T, et al., 2014)Applications of pI Determination
cIEF is a versatile tool with numerous applications across research, development, and quality control, including:
- Protein Characterization: Determination of pI to study protein stability, charge heterogeneity, and post-translational modifications (PTMs).
- Biopharmaceutical Development: Assessment of recombinant proteins, monoclonal antibodies, and biosimilars for charge variants and formulation optimization.
- Vaccine and Peptide Analysis: Characterization of vaccines, peptides, and other biologics to ensure consistency and compliance with regulatory standards.
Sample Requirements for pI Determination
- Sample Type: Proteins, peptides, recombinant proteins, monoclonal antibodies, and other amphoteric biomolecules.
- Sample Volume: ≥5-10 µL (Larger sample volumes can be accommodated if necessary).
- Sample Concentration: Protein concentration should generally be in the range of 0.1-2 mg/mL. Very dilute samples may need to be concentrated for optimal results.
- Buffer Composition: Samples should be prepared in a buffer that is compatible with cIEF, usually containing ampholytes, stabilizers, and pI markers.
- Additives: Optional additives such as reducing agents, detergents, or denaturants may be used depending on the sample type and the desired outcome.
Advantages of our Isoelectric point determination with cIEF:
- Rich experience in pI determination.
- High sensitivity and accuracy to detect proteins with different post-translational modifications.
- Rapid turnaround time to provide comprehensive report.
- Customized service: according to you sample type and sample size, optimized protocols will be deployed.
We provide but are not limited to:
- Isoelectric point determination of proteins
- Separation of proteins and peptides
FAQ
Q: Can cIEF be used for proteins that are difficult to solubilize or prone to aggregation?
A: Yes, cIEF can accommodate proteins with challenging solubility or aggregation properties. Creative Proteomics uses stabilizers and optimized buffers to maintain sample integrity. For complex proteins like membrane proteins or hydrophobic peptides, customized protocols ensure accurate analysis.
Q: Can cIEF analyze proteins with multiple isoforms or charge variants?
A: Yes, cIEF resolves proteins with multiple isoforms or charge variants by separating them based on isoelectric points along a pH gradient. It is well-suited for evaluating monoclonal antibody heterogeneity, identifying charge-related impurities, and analyzing isoform distribution in recombinant proteins.
Q: Does temperature affect cIEF analysis?
A: Temperature plays a critical role in cIEF as it can influence protein stability, the formation of the pH gradient, and focusing efficiency. Variations in temperature may alter the pI of the proteins or affect ampholyte behavior. Creative Proteomics uses temperature-controlled instruments to ensure consistency and reproducibility in all analyses.
Q: Can cIEF be used for comparative studies of protein variants?
A: Yes, cIEF is an ideal tool for comparative studies, such as:
Identifying subtle pI shifts due to single amino acid mutations.
Evaluating the impact of PTMs like phosphorylation or glycosylation.
Comparing biosimilars with their reference biologics to ensure consistency.
Q: What additives can be used to optimize cIEF for challenging samples?
A: Depending on the sample, various additives can be included, such as:
Denaturants (e.g., urea): For resolving complex or aggregated proteins.
Reducing agents (e.g., DTT): To break disulfide bonds and simplify analysis of native proteins.
Stabilizers: To prevent aggregation or degradation during focusing.
Case Study
Case: Characterization of recombinant human granulocyte colony stimulating factor (rHuG-CSF) by capillary zone electrophoresis, capillary isoelectric focusing electrophoresis and electrospray ionization mass spectrometry
Background
This study explores advanced methods for characterizing recombinant human granulocyte colony-stimulating factor (rHuG-CSF), a vital therapeutic protein, addressing challenges in ensuring its purity, identity, and structural integrity in pharmaceuticals. Traditional methods like bioassays and basic electrophoresis often fail to distinguish subtle structural and functional differences. The study emphasizes combining capillary zone electrophoresis (CZE), capillary isoelectric focusing electrophoresis (cIEF), and electrospray ionization mass spectrometry (ESI-MS) for a comprehensive analysis of rHuG-CSF, highlighting the significance of these tools in ensuring product quality.
Methods
- CZE processes
Utilized to assess purity and identify glycoforms based on the presence of acetylneuraminic acids in rHuG-CSF.
High sensitivity to detect active proteins amidst additives like human serum albumin (HSA).
- cIEF processes
Determined pI values to analyze protein charge heterogeneity.
Employed standard markers (ribonuclease A and CCK peptide fragment) for calibration.
Required sample desalting to eliminate interferences in pI determination.
- ESI-MS processes
Measured molecular weight with high accuracy to confirm the protein's identity.
Identified potential modifications and impurities by evaluating molecular weight deviations.
The resolution (2000 FWHM) limited the detection of minor variations in molecular mass.
Results
- cIEF findings:
The non-glycosylated rHuG-CSF bulk sample displayed three peaks with distinct pI values (6.75, 6.02, and 5.09).
GRAN300 injection, a related product, exhibited a single peak with a pI of 6.10.
The differences in pI indicated structural variations, such as sequence alterations or post-translational modifications.
- ESI-MS findings:
The observed molecular weight of rHuG-CSF (18,801 Da) closely matched the theoretical value.
The resolution of the MS system (2000 FWHM) could not detect minor molecular differences, such as amino acid substitutions with mass changes <9.4 Da.
Post-translational modifications larger than 10 Da were ruled out for the three CIEF peaks.
- Integrated Analysis:
The combined data from CIEF and ESI-MS suggested heterogeneity in the rHuG-CSF bulk sample.
High-resolution CIEF-ESI-MS was deemed necessary for detailed analysis of the protein's structural variations.
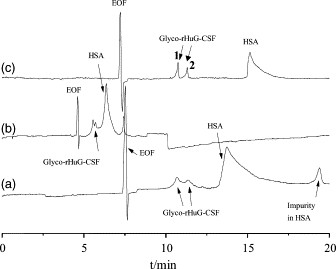 Figure 2. The separation of GRANOCYTE injections in different buffers: (a) 50 mM Tricine, pH 8.0; (b) 50 mM Tricine containing 20 mM NaCl, pH 8.0; (c) 50 mM Tricine containing 20 mM NaCl and 2.5 mM DAB, pH 8.0.
Figure 2. The separation of GRANOCYTE injections in different buffers: (a) 50 mM Tricine, pH 8.0; (b) 50 mM Tricine containing 20 mM NaCl, pH 8.0; (c) 50 mM Tricine containing 20 mM NaCl and 2.5 mM DAB, pH 8.0.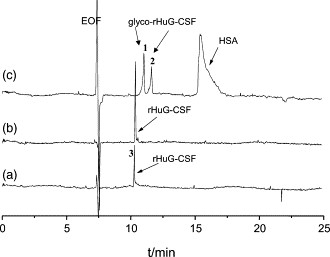 Figure 3. Capillary zone electropherograms of rHuG-CSF separated under the conditions of Fig. 1. Samples were non-glycosylated rHuG-CSF bulk sample (a), GRAN300 injections (b), and GRANOCYTE injections (c), respectively.
Figure 3. Capillary zone electropherograms of rHuG-CSF separated under the conditions of Fig. 1. Samples were non-glycosylated rHuG-CSF bulk sample (a), GRAN300 injections (b), and GRANOCYTE injections (c), respectively.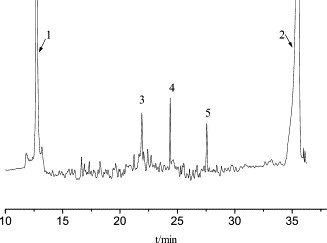 Figure 4. Capillary isoelectric focusing electropherogram of the non-glycosylated rHuG-CSF bulk sample.
Figure 4. Capillary isoelectric focusing electropherogram of the non-glycosylated rHuG-CSF bulk sample.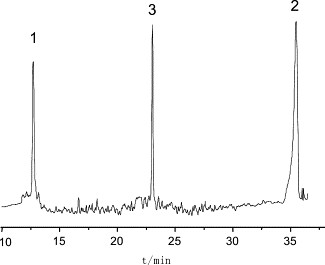 Figure 5. Capillary isoelectric focusing electropherogram of GRAN300.
Figure 5. Capillary isoelectric focusing electropherogram of GRAN300.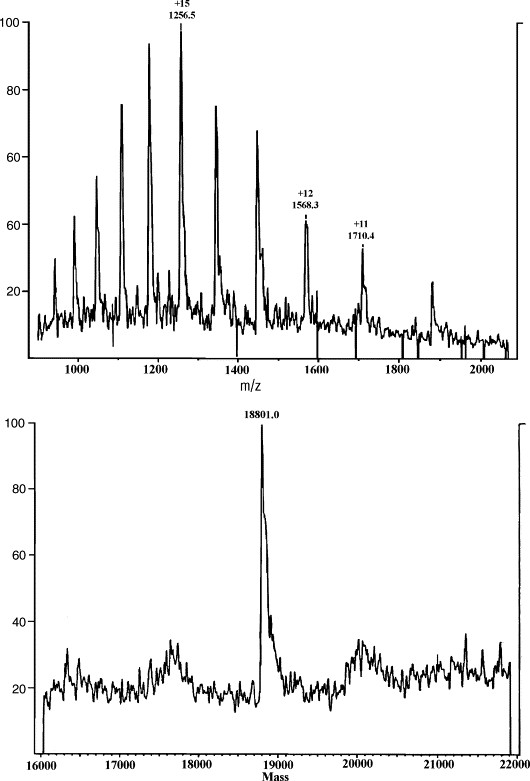 Figure 6. Mass spectrometry of rHuG-CSF. The top is the electrospray ionization (ESI) mass spectrum and the bottom is its deconvoluted mass spectrum.
Figure 6. Mass spectrometry of rHuG-CSF. The top is the electrospray ionization (ESI) mass spectrum and the bottom is its deconvoluted mass spectrum.References
- Righetti P G. Determination of the isoelectric point of proteins by capillary isoelectric focusing. Journal of chromatography A, 2004, 1037(1-2): 491-499. DOI: 10.1016/j.chroma.2003.11.025
- Audain E, Ramos Y, et al. Accurate estimation of isoelectric point of protein and peptide based on amino acid sequences. Bioinformatics, 2016, 32(6): 821-827. DOI: 10.1093/bioinformatics/btv674
- Kristl T, et al. Principles and Applications of Capillary Isoelectric Focusing. 2014. 45 p. https://www.agilent.com/cs/library/primers/public/5991-1660EN.pdf
- Zhou G H, et al. Characterization of recombinant human granulocyte colony stimulating factor (rHuG-CSF) by capillary zone electrophoresis, capillary isoelectric focusing electrophoresis and electrospray ionization mass spectrometry. Journal of pharmaceutical and biomedical analysis, 2004, 35(3): 425-432. DOI:
10.1016/j.jpba.2004.02.003
Related Services
Support Documents
KNOWLEDGE CENTER
KNOWLEDGE CENTER












