- Services
- Demo
- Case Study
- FAQ
- Related Services
- Support Documents
- Inquiry
Monoclonal antibodies (mAbs) are now central to the treatment of cancer, autoimmunity, infectious diseases, and more. As the fastest growing class of biopharmaceuticals, mAbs demand rigorous analytical characterization to meet regulatory standards and ensure product safety, efficacy, and stability.
At Creative Proteomics, we've built a world-class LC-MS platform specifically tailored for intact antibody analysis. With over 20 years of experience and continual investment in innovation, our team supports biopharma and CRO partners with high-sensitivity, high-resolution data for complex protein therapeutics.
What Makes LC-MS Ideal for Antibody Characterization?
Liquid Chromatography–Mass Spectrometry (LC-MS) is now the gold standard for analysing intact mAbs. Its advantages include:
- Compatibility with a wide range of LC columns and validated workflows
- High sensitivity and resolution for mass measurement
- Ability to detect critical quality attributes in a single run
Our LC-MS service is frequently used to:
- Confirm antibody sequence integrity
- Determine intact molecular weight with <10 ppm accuracy
- Detect post-translational modifications (PTMs), including glycosylation and oxidation
- Identify binding epitopes via peptide mapping
- Monitor antibody degradation or heterogeneity during formulation
Our Optimized LC-MS Workflow: From Sample to Insight
We've designed a robust, reproducible workflow that yields high-quality data across antibody formats.
Five Core Stages:
- Experimental Design – Tailored to your antibody class and application
- Sample Preparation – Includes purification, buffer exchange, and enzymatic digestion
- LC-MS Analysis – Performed on state-of-the-art high-resolution MS platforms
- Data Processing – Raw data deconvolution, annotation, and peak assignment
- Report Delivery – Interpretable result reports plus optional bioinformatics summaries
We also support heavy/light chain reduction and subunit-level analysis using native or denaturing conditions.
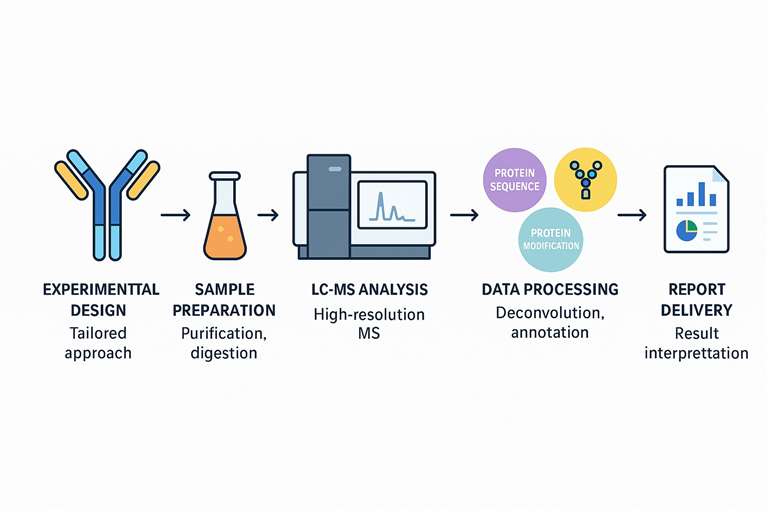
Built for Next-Generation Antibody Platforms
Our LC-MS approach keeps pace with today's innovations in antibody engineering, including:
- Bispecifics and antibody-drug conjugates (ADCs)
- Fc-fusion proteins
- PEGylated antibody fragments
- Complex glycoform variants
To ensure sensitivity and quantification accuracy, we focus on high-efficiency sample recovery and affinity enrichment strategies during pre-analytical steps.
What Can You Analyze with This Service?
Creative Proteomics' LC-MS analysis is used by clients worldwide to study:
✅ Primary sequence confirmation
✅ Relative glycoform quantification
✅ Site-specific PTMs (oxidation, deamidation, C-terminal lysine variants)
✅ Epitope mapping for antibody–antigen interactions
Why Choose Creative Proteomics?
- High-sensitivity instrumentation for intact antibody detection
- Optimized workflows for peptide recovery and digestion
- High-throughput capacity for screening and QC batches
- Cost-effective packages tailored to mAb pipelines
- Expert support from design to data interpretation
- MALDI-TOF-MS + ESI-MS
| Feature | Creative Proteomics | Traditional Methods | Generic MS Labs |
|---|---|---|---|
| Intact Mass Accuracy | ✅ <10 ppm | ❌ Not supported | ⚠️ Variable |
| Glycoform Quantification | ✅ Yes | ❌ No | ⚠️ Optional |
| Turnaround Time | ✅ Fast, scalable | ⚠️ Moderate | ⚠️ Varies |
| Regulatory Readiness | ✅ Submission-grade data | ❌ Not suitable | ⚠️ Requires validation |
| Scientific Support Included | ✅ Yes | ❌ No | ⚠️ Limited |
Who Is This For?
This Service Is Ideal For:
Biopharma scientists validating mAb identity during clone selection
CMC and QA/QC teams confirming consistency across production batches
CROs and CDMOs seeking a reliable LC-MS partner for antibody analytics
Protein engineers characterizing Fc mutants or bispecific constructs
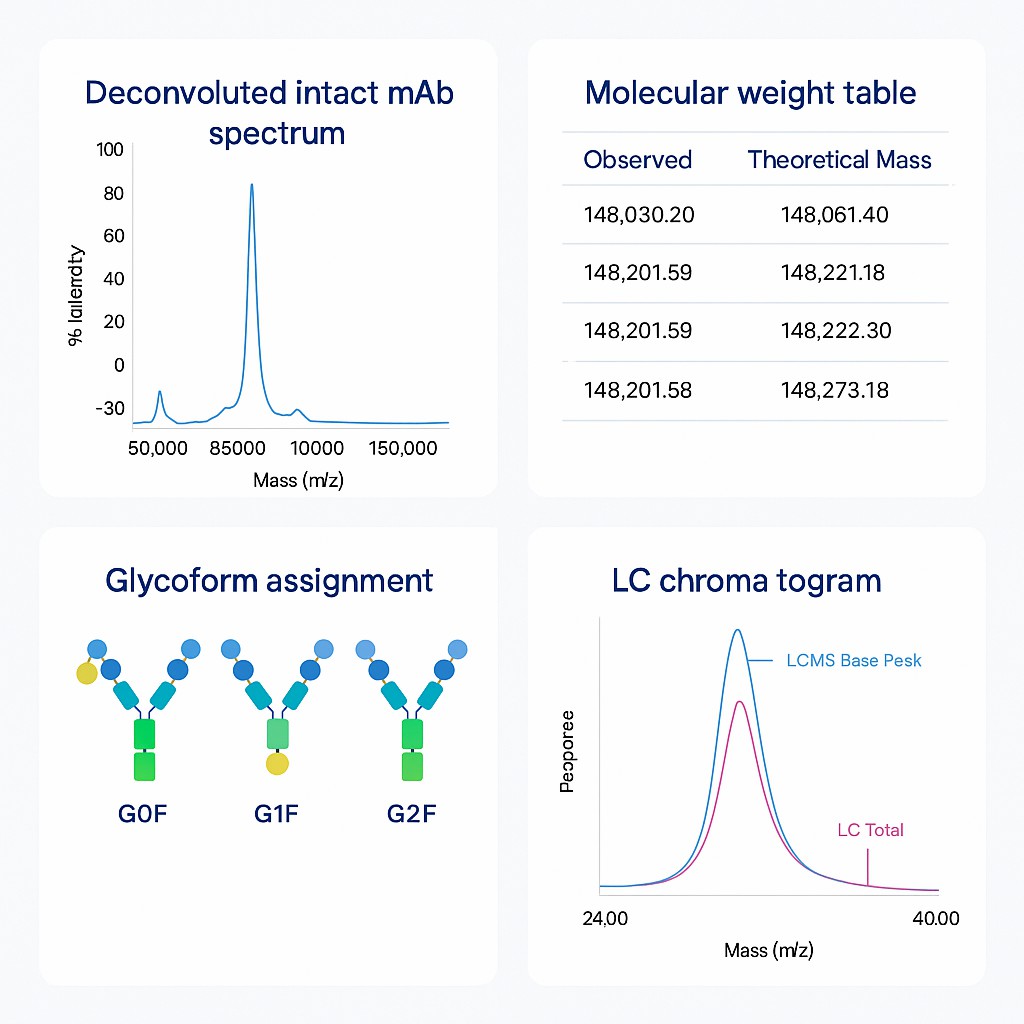
What You'll Receive
Your Report Will Include:
- Intact mAb spectrum (raw and deconvoluted)
- Molecular weight data: observed vs. theoretical
- Glycoform profiles (if applicable)
- Peak annotation and retention time
- Optional: Peptide map overlay or subunit analysis (on request)
Customer Testimonials
What Our Clients Say:
"We needed intact mAb mass results under a tight deadline. Creative Proteomics delivered, and their report was submission-ready."
— Director of Biologics, U.S. Biotech Company
"This service helped us validate clone integrity before cell line development. Very responsive team."
— Senior Scientist, Biomanufacturing R&D
Let's Advance Your Antibody Pipeline with LC-MS
Whether you're validating a new clone, comparing biosimilar batches, or investigating sequence variants—our LC-MS intact antibody analysis helps you move from uncertainty to confidence.
Demo
Case Study: LC-MS Characterization of an Asymmetric Antibody
Title: LC-MS characterization and purity assessment of a prototype asymmetric antibody
Authors: Doneanu CE, Xenopoulos A, Fadgen K, Murphy J, Skilton SJ, Prentice H, et al.
Journal: mAbs
Year: 2013
DOI / Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3851224/
Background
Bispecific antibodies, which bind two distinct antigens, offer enhanced therapeutic potential compared to conventional monoclonal antibodies. However, their development presents unique challenges—particularly with respect to correct heavy chain pairing and the presence of mispaired homodimeric forms. Conventional analytical techniques often lack the resolution and specificity needed to distinguish these subtle product-related impurities.
This study aimed to establish a high-resolution LC-MS method to monitor heterodimer assembly and detect homodimer contaminants in an asymmetric antibody molecule.
Methodology
The researchers developed a workflow combining intact protein mass analysis and peptide mapping using LC-MS platforms:
- Intact Mass Analysis: The antibody was enzymatically deglycosylated and analyzed via LC-MS to determine the mass distribution of heavy/light chains and identify molecular heterogeneity.
- Peptide Mapping: Using Lys-C digestion and LC-MS^E (MS with data-independent acquisition), they validated sequence integrity and detected post-translational modifications, including C-terminal truncations.
- Purity Assessment: A quantitative method was devised by spiking known concentrations of homodimer standards into deglycosylated heterodimers, simulating different impurity levels for accurate quantification.
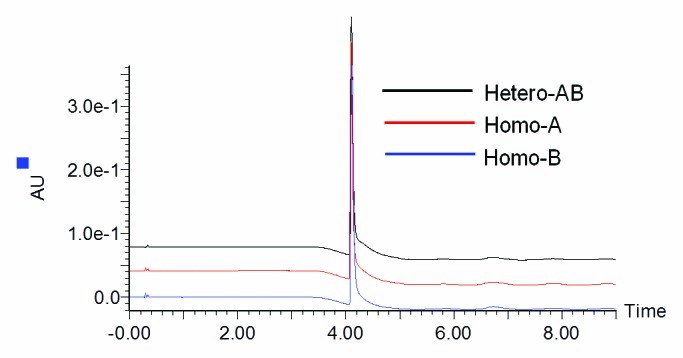 Characterization of MAb1 and homodimeric controls by LC-MS
Characterization of MAb1 and homodimeric controls by LC-MSKey Results
- The LC-MS method successfully detected homodimer impurities as low as 2%, even in the presence of co-eluting species and half-antibody forms.
- Truncated species were quantifiable at levels below 0.6%, demonstrating high sensitivity for low-abundance variants.
- The platform was found to be suitable for purity screening during clone selection and process development in bispecific antibody programs.
Conclusion
This study demonstrated that intact mass analysis by LC-MS, combined with high-resolution peptide mapping
FAQ
Q: What types of monoclonal antibodies can be analysed with this service?
A: We support standard IgG subclasses, bispecific antibodies, Fc-fusion proteins, PEGylated fragments, and ADCs.
Q: Do I need to provide buffer-free or purified samples?
A: High-purity samples are preferred. If your antibody is in PBS or Tris buffer, we offer buffer exchange and cleanup prior to LC-MS analysis.
Q: How accurate is your molecular weight measurement?
A: We routinely achieve <10 ppm mass accuracy, enabling precise detection of glycoform shifts, truncations, or degradation.
Q: Can I analyse intact mass and glycosylation in one run?
A: Yes. Our LC-MS platform allows simultaneous profiling of intact mAb molecular weight and relative glycoform distribution.
Q: Is this service applicable for stability or comparability studies?
A: Absolutely. Our workflow is frequently used for batch-to-batch comparison, forced degradation studies, and biosimilarity testing.
Related Services
Support Documents
KNOWLEDGE CENTER
Molecular Weight Characterization - Comparison of MALDI and ESI











