- Services
- FAQ
- Case Study
- Related Services
- Support Documents
- Inquiry
What is Protein Purity and Homogeneity?
Protein purity refers to the proportion of the target protein in a sample relative to contaminants, impurities, or degradation products. Achieving high protein purity is essential for ensuring that the protein functions as intended in research or therapeutic applications. Protein homogeneity refers to the consistency and uniformity of the protein sample, ensuring that all molecules are of the same size, structure, and functional state. Homogeneity is crucial for achieving reliable experimental results, particularly when using proteins in structural biology studies or therapeutic formulations.
In biotechnology and pharmaceuticals, the purity and heterogeneity of antibodies, protein therapeutics, and recombinant proteins must be determined to ensure their biological activity. Contaminants and modifications during production can alter the drug's effectiveness. Accurate, sensitive methods for evaluating protein purity and homogeneity are essential for ensuring high-quality, safe, and effective protein drugs.
At Creative Proteomics, we are committed to providing the highest level of protein analysis to ensure the purity and homogeneity of your samples. Our advanced techniques, combined with our extensive expertise, deliver accurate, reliable results that meet the demands of your research and development processes.
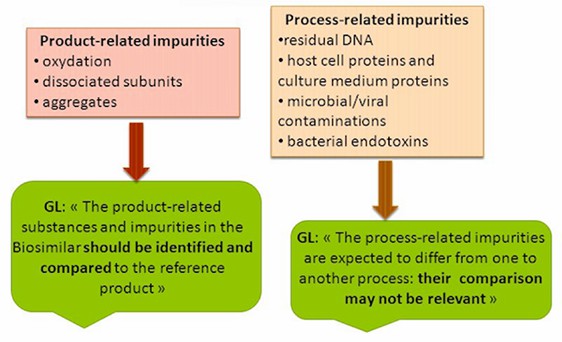 Figure 1. Protein drug impurities.
Figure 1. Protein drug impurities.We Can Provide (but not limited to):
- Purity and Impurity Analysis (SDS-PAGE)
- Protein Polymer Analysis (HPLC)
- Protein Fragment Analysis (HPLC)
- Protein Isoform Analysis (cIEF)
- Host Cell Proteins Analysis
- Host Cell Residual DNA Analysis
- Antibody Homogeneity Analysis Service
- Vaccine Homogeneity Analysis Service
- PEGylated Protein Homogeneity Analysis Service
Technology Platform of Protein Purity and Homogeneity Analysis
Our Protein Purity and Homogeneity Analysis service leverages cutting-edge technologies and platforms to provide highly accurate, reliable, and reproducible results. These technologies include:
Mass Spectrometry (MS): Our MS platforms, including LC-MS and MALDI-TOF, provide detailed information on protein structure, contaminants, and post-translational modifications with high sensitivity.
Dynamic Light Scattering (DLS): For real-time monitoring of protein aggregation and size distribution in solution.
Chromatographic Techniques: We utilize both SEC and HPLC for separation and purity assessment, separating proteins based on their size, providing detailed information about the molecular weight distribution and the presence of aggregates or oligomers..
SDS-PAGE: SDS-PAGE and other gel electrophoresis techniques are employed for visualizing protein purity and detecting degradation or impurities.
Why Choose Our Protein Purity and Homogeneity Analysis Service
- High Sensitivity and Accuracy: Our state-of-the-art instruments provide precise, reproducible measurements with unmatched sensitivity, even for low-abundance proteins or complex mixtures.
- Comprehensive and Multimodal: Employing a variety of complementary techniques that address the full spectrum of purity and homogeneity challenges, ensuring thorough characterization of your protein sample.
- Customization: Tailoring our analysis to meet your specific requirements, providing personalized reports and consultations for each project.
- Quick Turnaround: Offering fast and efficient analysis without compromising on quality.
- Regulatory Compliance: Adhering to rigorous quality control and regulatory standards, ensuring that our services meet the requirements of the pharmaceutical, biotechnology, and academic sectors.
Applications of Protein Purity and Homogeneity Analysis
- Therapeutic Protein Development: Ensuring the purity and homogeneity of biopharmaceuticals, monoclonal antibodies, and vaccines.
- Structural Biology: Verifying protein integrity before structural analysis using NMR, X-ray crystallography, or cryo-EM.
- Preclinical and Clinical Research: Validating protein samples for assays, toxicity studies, and drug screening.
- Biotechnology: Supporting the production and purification of recombinant proteins used in diagnostics, vaccines, and industrial applications.
- Protein Engineering: Ensuring that engineered proteins are pure, homogeneous, and functional for use in therapeutic applications.
Sample Requirements
- Sample Type: Pure protein or protein mixtures in solution, such as purified recombinant proteins, antibodies, enzymes, or biologic therapeutics.
- Concentration: Minimum concentration of 0.1 mg/mL. Higher concentrations may be required for certain analyses, such as mass spectrometry.
- Volume: At least 100 µL of the sample is recommended for most techniques. Larger volumes may be needed for specific analyses like SEC.
- Buffer Conditions: Samples should be provided in a buffer that is compatible with the chosen analytical technique (e.g., phosphate-buffered saline, Tris buffer). Avoid high salt concentrations or detergents unless specifically requested.
- Storage: Protein samples should be stored at -80°C or in liquid nitrogen if not immediately analyzed. Samples should be free of particulates and aggregates before submission.
FAQ
Q: What is the detection limit for impurities, and can your service detect low-abundance contaminants?
A: Our service is designed to detect impurities at very low concentrations, with the detection limit varying depending on the method:
- SDS-PAGE can identify major contaminants down to about 1-2% of the total protein content.
- HPLC can detect contaminants at concentrations as low as 0.1-0.5%.
- Mass Spectrometry (MS) provides the highest sensitivity, allowing for the detection of trace amounts of contaminants, even at the sub-nanogram level.
- SEC and DLS can identify aggregates and non-homogeneous populations, with sensitivity down to nanomolar concentrations.
Q: Do you provide a service for comparing the purity and homogeneity of protein samples across different production batches?
A: Yes, we offer comparative analysis of protein samples across different production batches. By analyzing multiple batches of your protein, we can assess:
- Batch-to-batch consistency in terms of purity and homogeneity.
- Identifying trends in the presence of contaminants or variations in protein aggregation.
- Assessment of PTMs that may vary with production conditions or storage.
This service is especially useful for ensuring the reproducibility and stability of biotherapeutics over time or across different production methods.
Q: Can you handle large-scale sample analysis, such as high-throughput screening of protein variants?
A: Yes, we offer high-throughput screening capabilities for large-scale analysis of protein variants. This service is designed to process and analyze multiple samples in parallel, providing rapid assessments of purity and homogeneity across a large number of variants. We can integrate automated systems to streamline the process, ensuring that you receive consistent and reliable data for each sample. This is particularly beneficial for projects involving protein engineering, drug discovery, or biomarker development where high-volume testing is necessary.
Case Study
Case: Purity determination and uncertainty evaluation of L-selenium-methylselenocysteine
Background
This study addresses the need for developing a Certified Reference Material (CRM) for L-selenium-methylselenocysteine (L-SeMC), a source of organic selenium with higher biological activity and bioavailability compared to inorganic forms like sodium selenite. The purity of L-SeMC is crucial for its standardization as a supplement in food and pharmaceuticals, but no solid CRM for L-SeMC has been developed. The article discusses various methods to determine the purity of L-SeMC and provides an uncertainty evaluation.
Methods
- HPLC (High-Performance Liquid Chromatography): Used to confirm the presence and purity of L-SeMC by comparing its chromatographic peak with that of a known methyl-selenocysteine CRM, identifying potential impurities or contaminants.
- NMR (Nuclear Magnetic Resonance): Employed to analyze the chemical shifts in the hydrogen and carbon spectra of L-SeMC, providing detailed structural information to confirm the compound's identity and purity.
- FTIR (Fourier Transform Infrared Spectroscopy): Used to identify functional groups such as amine, carboxyl, and methyl groups, confirming the presence of key molecular features of L-SeMC.
- DSC (Differential Scanning Calorimetry): Analyzed the thermal behavior of L-SeMC to assess its melting point and potential impurities. However, it was determined unsuitable for purity determination due to the presence of endothermic peaks unrelated to impurities.
- Mass Balance Method: Applied to quantify impurities by subtracting the amounts of all impurity components from the total sample, using techniques like gas chromatography and HPLC for impurity identification.
- ICP-MS (Inductively Coupled Plasma Mass Spectrometry): Used to identify selenium and other inorganic element impurities, confirming the selenium content in the sample and identifying minor elemental contaminants.
- qNMR (Quantitative NMR): A primary method for purity determination, using maleic acid as an internal standard, providing traceability and accurate purity measurement.
Results
Structure Determination:
- HPLC: Confirmed the presence of L-SeMC by matching its chromatographic peak to that of the CRM and detecting no impurities or D-SeMC.
- NMR: Both 1H and 13C NMR spectra confirmed the molecular structure of L-SeMC with consistent chemical shifts reported in literature.
- FTIR: The FTIR spectrum confirmed the presence of functional groups such as carboxyl, amine, and methyl groups, consistent with L-SeMC's structure.
Homogeneity and Stability:
- The uniformity test showed no significant variation in purity between samples, indicating that L-SeMC is uniform.
- Stability tests under various temperature conditions (37°C, 50°C, −20°C, 4°C, and 25°C) over short- and long-term periods demonstrated no significant degradation, suggesting that L-SeMC maintains its integrity over time.
- The uncertainty associated with stability was low, and the sample can be stored and transported under typical conditions for over a year without significant changes.
Purity Determination:
- DSC: The DSC method showed two endothermic peaks but was unsuitable for purity determination due to interference from recrystallization processes.
- Mass Balance Method: Determined the purity of L-SeMC to be nearly 100%, with very low levels of volatile and non-volatile impurities detected. The uncertainty in this method was calculated to be 0.46%.
- ICP-MS: Detected a low level of inorganic element impurities, but their presence did not significantly affect the sample's overall purity.
- qNMR: Provided a purity of 99.70%, consistent with the mass balance method, and with lower uncertainty (0.16%) compared to the mass balance method.
Fixed Value and Uncertainty Evaluation:
- By combining the mass balance method and qNMR results, the fixed purity value for L-SeMC was determined to be 99.70%, with an expanded uncertainty of 0.28%. The results showed that both methods are reliable and can be used for purity determination in L-SeMC CRM development.
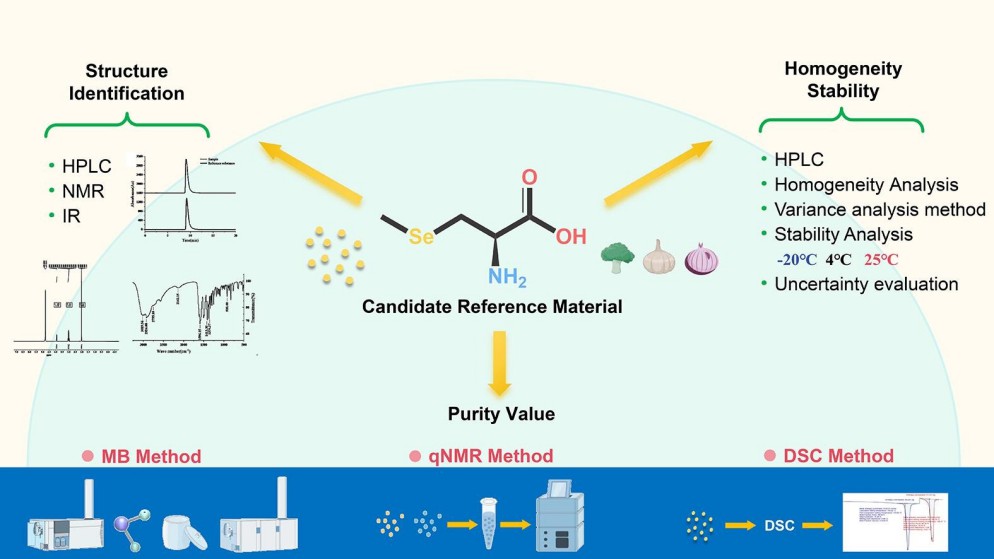 Figure 2. Graphical abstract for Purity determination and uncertainty evaluation of L-selenium-methylselenocysteine.
Figure 2. Graphical abstract for Purity determination and uncertainty evaluation of L-selenium-methylselenocysteine.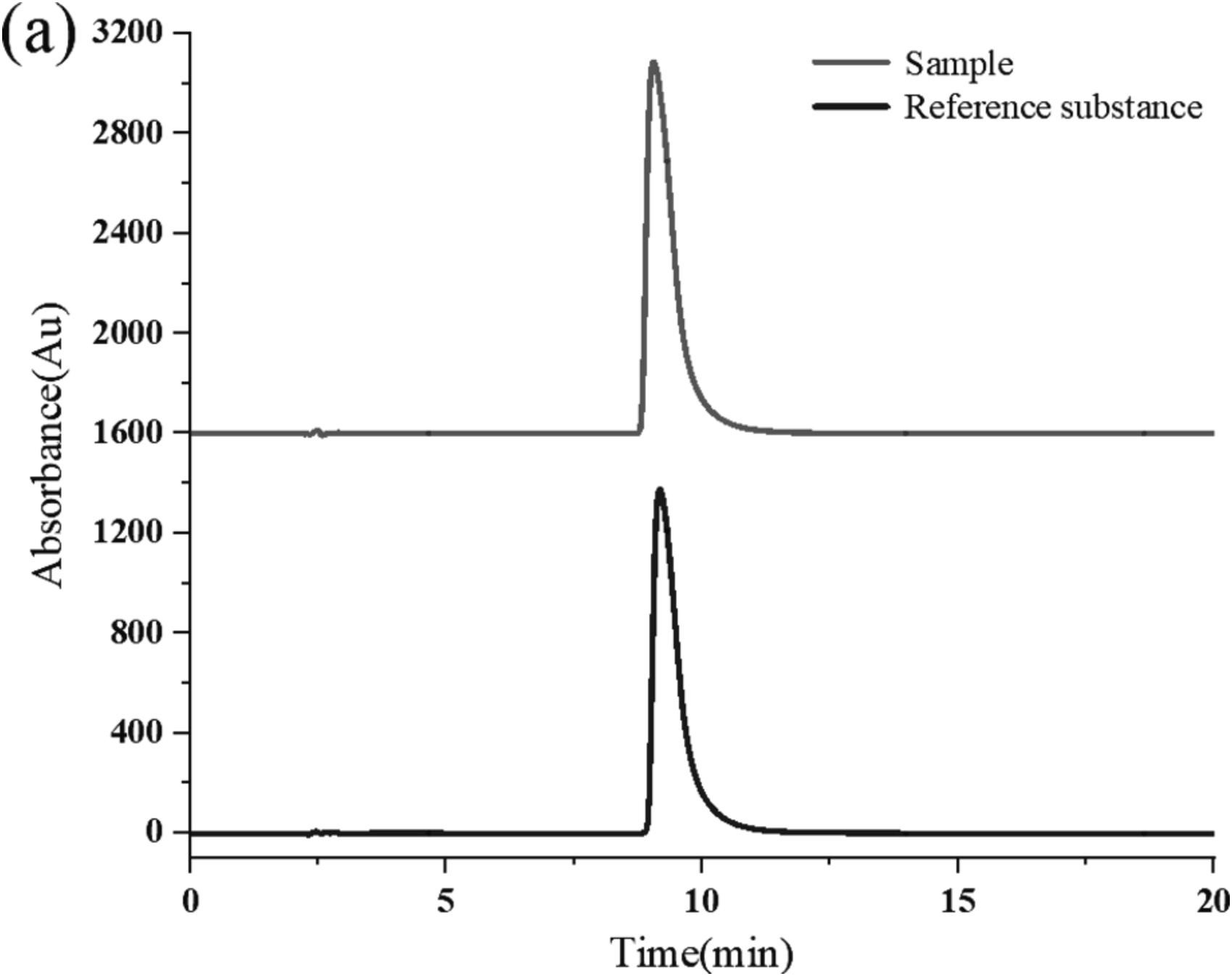 Figure 3. Liquid chromatogram of L-SeMC.
Figure 3. Liquid chromatogram of L-SeMC.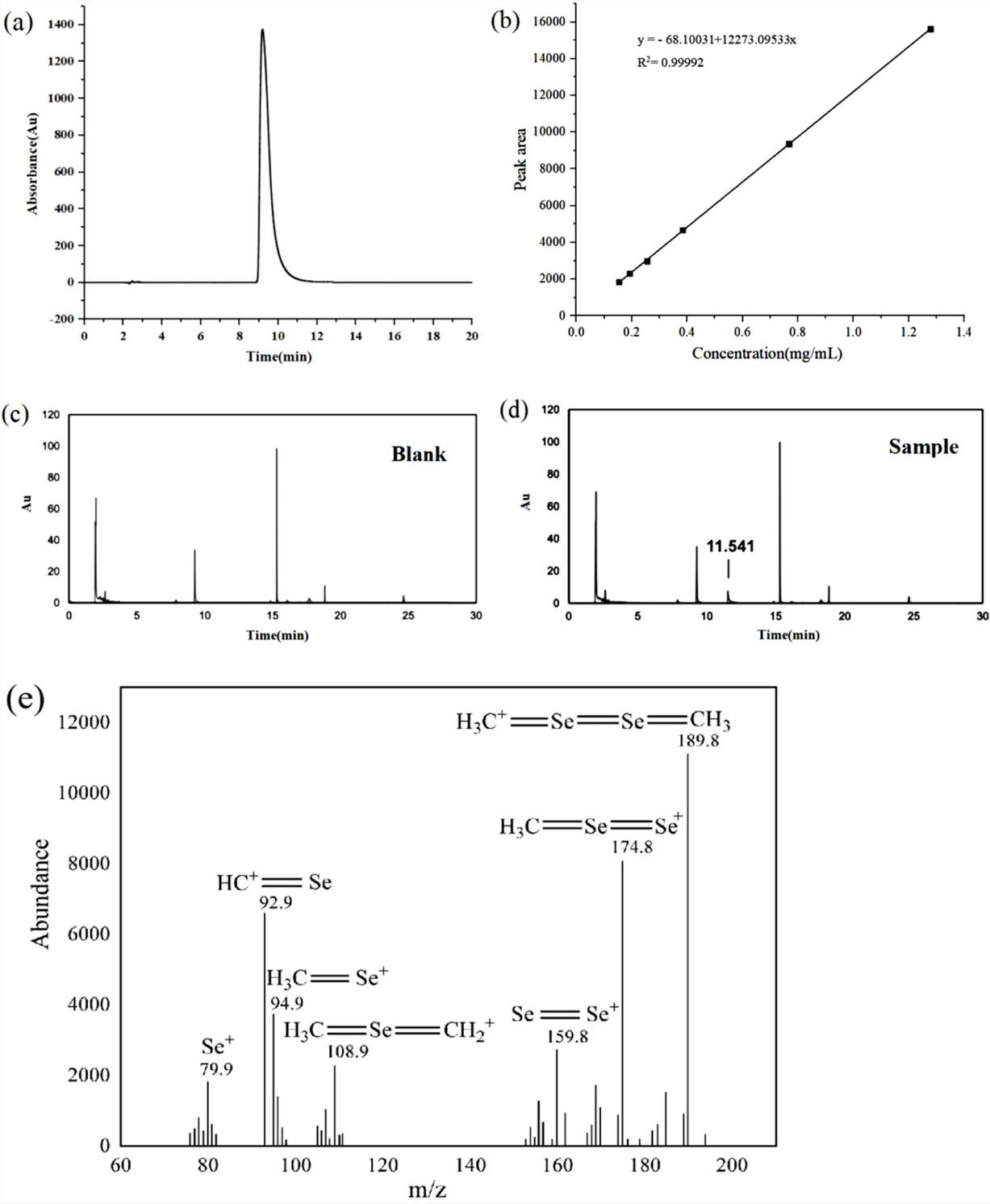 Figure 4. HPLC chromatogram of L-SeMC sample.
Figure 4. HPLC chromatogram of L-SeMC sample.References
- Tang X, et al. Purity determination and uncertainty evaluation of L-selenium-methylselenocysteine. Microchemical Journal, 2024, 207: 112154. DOI: 10.1016/j.microc.2024.112154
- Raynal B, et al. Quality assessment and optimization of purified protein samples: why and how?. Microbial cell factories, 2014, 13: 1-10. DOI: 10.1186/s12934-014-0180-6
Related Services
Support Documents













