- Services
- FAQ
- Case Study
- Related Services
- Support Documents
- Inquiry
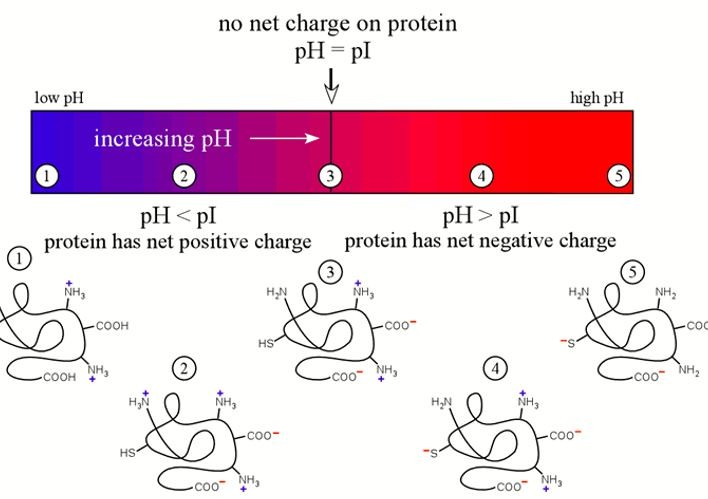
What is Isoelectric Point (pI)?
The isoelectric point (pI) is the pH at which a molecule has no net charge, representing a balance between its positive and negative charges. This key property of amphoteric molecules like amino acids, peptides, and proteins is influenced by the ionizable groups, particularly the amino and carboxyl groups in proteins.
The isoelectric point (pI) significantly affects the behavior of biomolecules in solution, impacting their solubility, interactions, and stability. Below the pI, molecules have a net positive charge, which can enhance solubility in certain environments. Above the pI, molecules acquire a net negative charge, influencing their interactions with other biomolecules and affecting stability and function in various conditions.
Creative Proteomics offers accurate and fast pI determination following ICH Q6B guidelines during drug development.
How to Detect pI?
Isoelectric Focusing (IEF)
IEF separates proteins by their pI within a pH gradient with precise, high-resolution. Proteins migrate to the pH matching their pI, stopping movement as they reach a neutral charge. This technique is highly sensitive and accurate, suitable for proteins, peptides, and amphoteric molecules.
Capillary Isoelectric Focusing (cIEF)
This technique is used for the determination of pI in a capillary setup. Proteins or peptides migrate through a narrow capillary filled with a pH gradient buffer. The sample moves until it reaches a point where the pH matches its pI, and the molecule stops migrating. Capillary electrophoresis offers rapid analysis with minimal sample usage and is particularly useful for small sample sizes.
pH Titration
This classical method involves the gradual adjustment of pH while monitoring the molecule's charge state. The pI is determined at the inflection point of the titration curve, where the molecule's net charge is zero. Though less precise than IEF or CE, pH titration is a reliable and straightforward technique for certain applications.
| Method | Advantages | Disadvantages |
|---|---|---|
| IEF | - High resolution. - Can separate complex mixtures of proteins with similar pIs. - Directly measure. |
- Requires large amounts of sample. - Needs specialized equipment. - Labor-intensive and time-consuming. |
| cIEF | - High resolution. - Faster than traditional IEF. - Requires minimal sample consumption. - Automated, high-throughput option. |
- Needs specialized equipment required. - Limited by sample size. - More complex interpretation of results for non-experts. |
| pH Titration | - Simple and cost-effective, requiring minimal specialized equipment. - Widely applicable for a range of molecules. - Suitable for both small and large sample sizes. |
- Lower precision . - Time-consuming. - Not suitable for samples with complex mixtures or unknown compositions. - Requires careful monitoring of pH changes. |
Applications of pI Determination
- Protein Purification: By understanding the pI, proteins can be separated and purified efficiently using IEF, ion-exchange chromatography, and precipitation techniques. The pI helps in identifying the optimal pH for maximal purification.
- Proteomics: pI determination is vital for protein identification, characterization, and quantification. For example, isoelectric focusing in 2-DE can separates complex protein mixtures.
- Enzyme Characterization: The pI of enzymes affects their catalytic properties, substrate specificity, and stability, aiding in the optimization of enzyme-based processes and biotechnological applications.
- Biopharmaceutical Development: pI helps in predicting solubility, stability, and interactions in drug development, ensuring the quality and performance of biologic drugs.
- Antibody Development: pI determination of monoclonal antibodies and therapeutic proteins helps characterize charge variants, critical for understanding pharmacokinetics and therapeutic efficacy.
Sample Requirements for pI Determination
- Sample type: Pure proteins, peptides, or other biomolecules in aqueous solution or buffer.
- Protein concentration: Minimum of 0.5 mg/mL
- Purity: Greater than 90%
- Sample volume: At least 50 µL
Advantages of pI Determination Service
- Rich experience in pI determination.
- High sensitivity and accuracy to detect proteins with different post-translational modifications.
- Rapid Turnaround Time.
- Customized service: according to you sample type and sample size, optimized protocols will be deployed.
Creative Proteomics's experts can provide our customers with a precise pI determination for proteins and peptides samples. You can choose the technique for pI determination or discuss with our scientists. Clear and concise written reports including protocols and customized services will be offered during drug development.
FAQ
Q: Can you determine the pI of post-translationally modified proteins or peptides?
A: Yes, Creative Proteomics specializes in determining the pI of modified proteins and peptides. PTMs, like phosphorylation or glycosylation, can significantly alter pI. Our cIEF and IEF methods are highly effective in detecting small shifts in pI due to PTMs, allowing for precise characterization of modified proteins and peptides.
If you're interested in understanding how a PTM affects your protein's pI, we recommend cIEF, as it provides high sensitivity for these modifications.
Q: What if my protein is highly unstable – can you still determine the pI accurately?
A: For highly unstable proteins, we apply specialized handling and measurement protocols to preserve sample integrity. cIEF is particularly advantageous for such samples, as it requires minimal sample volume and short processing times, reducing exposure to destabilizing conditions. We also work closely with clients to adjust sample buffers, temperatures, and handling conditions to enhance protein stability throughout the analysis.
If you have a highly sensitive or unstable protein, please contact us for a customized assessment and to discuss any special requirements.
Q: How does pI determination help in assessing protein stability?
A: Determining the pI provides insights into a protein's solubility and aggregation behavior, which are key aspects of stability:
Solubility: At or near the pI, proteins are less soluble, which can lead to aggregation. Understanding the pI helps in adjusting buffer conditions away from this point to enhance solubility.
Aggregation Tendency: Proteins are more likely to aggregate at their pI due to reduced electrostatic repulsion, a critical consideration for formulation and storage.
Case Study
Case: Isoelectric point of free and adsorbed cytochrome c determined by various methods
Background
The isoelectric point (pI) of a protein is a critical biochemical property, representing the pH at which the protein has no net charge. Accurate determination of pI is essential, but traditional methods, such as free-boundary electrophoresis, are costly and complex, often requiring specialized laboratories and additional corrections for translational diffusion. Advances in technology have introduced other methods like dynamic light scattering, capillary electrophoresis, and isoelectric focusing (IEF) to measure pI. Despite their advantages, these methods have their limitations. For instance, IEF, though widely used, has accuracy limitations due to pH gradient inconsistencies and protein interactions with ampholytes. The study aims to address these limitations by comparing pI values derived from different techniques and discussing the limitations of computational models in accurately calculating protein electrostatics.
Methods
- Free-boundary electrophoresis: Utilized historically for pI determination, this technique was only briefly discussed here, given its complexity and technical demands.
- Dynamic light scattering: Used to measure zeta potential of proteins in solution, though high protein concentrations are required, increasing aggregation risk.
- Capillary electrophoresis: Involves measuring the velocity of proteins and determining pI by comparing peaks of protein movement relative to the electroosmotic flow. Adjustments to the capillary surface can reduce flow but cannot fully eliminate protein adsorption issues.
- IEF: IEF is identified as a standard technique but with challenges in determining precise pI due to issues like non-linearity in pH gradients. The microelectrophoresis method was used to analyze the electrophoretic mobility (IEP) of cytC adsorbed on colloidal particles, allowing for comparison to free protein values under controlled conditions.
- Protein Electrostatics Calculations: Software models are used to predict protein charge characteristics but face challenges in accounting for ion interactions and protein conformation changes in aqueous solutions, particularly with cytC.
Results
The study shows variability in pI values for cytC across different methods, with literature values ranging from pH 9.0 to 10.65. Results indicate that experimental pI values depend heavily on factors like protein conformation, ionic strength, and measurement techniques. Key findings include:
- IEF and Microelectrophoresis: The pI of cytC adsorbed on colloidal particles (pIμ) was close to that obtained through IEF, at approximately pH 9.35 and pH 9.44, respectively. This consistency suggests that colloidal adsorption methods may provide reliable pI values when ionic strength is minimized.
- Methodological Limitations: The article highlights the influence of experimental conditions on pI, including ion adsorption effects, protein aggregation, and the reliability of computational models. Traditional methods show limitations due to inaccurate pH gradients or protein-surface interactions, which skew results.
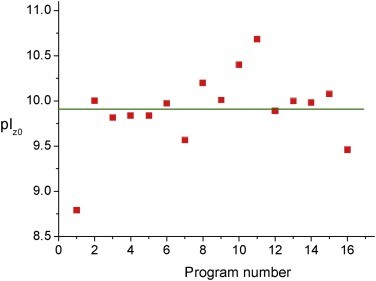 Figure 1. Point of zero charge pIz0 calculated by 16 programs with fixed pKa values of the ionizable groups of cytC polypeptide chain.
Figure 1. Point of zero charge pIz0 calculated by 16 programs with fixed pKa values of the ionizable groups of cytC polypeptide chain.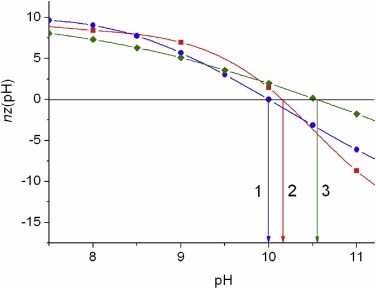 Figure 2. pH-dependence of the net charge nz of cytC globule calculated by Propka (curve 1, pInz 9.99), PHEMTO (curve 2, pInz 10,16), and Bluues (curve 3, pInz 10.55) using 3D-atom coordinates of crystallographic structure 1hrc of horse cytochrome c with molecular mass 12,360 kg/mol.
Figure 2. pH-dependence of the net charge nz of cytC globule calculated by Propka (curve 1, pInz 9.99), PHEMTO (curve 2, pInz 10,16), and Bluues (curve 3, pInz 10.55) using 3D-atom coordinates of crystallographic structure 1hrc of horse cytochrome c with molecular mass 12,360 kg/mol.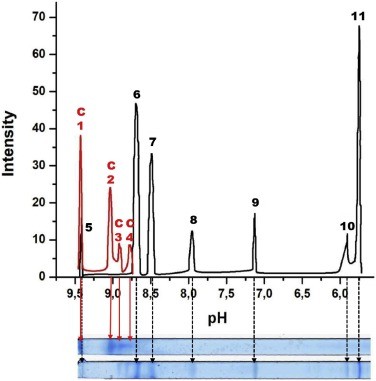 Figure 3. Two IPG strips with standard proteins (lower) and cytC (upper) and distribution of the optical density (ordinate) along the strips (abscissa).
Figure 3. Two IPG strips with standard proteins (lower) and cytC (upper) and distribution of the optical density (ordinate) along the strips (abscissa).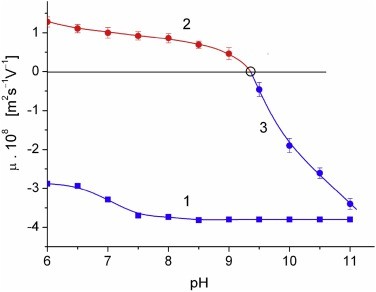 Figure 4. pH-dependences of the electrophoretic mobility μ of bare MM plates (curve 1) and cytC-MM particles under (curve 2) and above (curve 3) the recharging point at pH 9.35 (the cycle is value interpolated to μ = 0).
Figure 4. pH-dependences of the electrophoretic mobility μ of bare MM plates (curve 1) and cytC-MM particles under (curve 2) and above (curve 3) the recharging point at pH 9.35 (the cycle is value interpolated to μ = 0).References
- Righetti P G. Determination of the isoelectric point of proteins by capillary isoelectric focusing. Journal of chromatography A, 2004, 1037(1-2): 491-499. DOI: 10.1016/j.chroma.2003.11.025
- Ganapathy‐Kanniappan S. pI Determination of Native Proteins In Biological Samples. Current Protocols in Protein Science, 2019, 96(1): e85. DOI: 10.1002/cpps.85
- Pergande M R, Cologna S M. Isoelectric point separations of peptides and proteins. Proteomes, 2017, 5(1): 4. DOI: 10.3390/proteomes5010004
- Hristova S H, Zhivkov A M. Isoelectric point of free and adsorbed cytochrome c determined by various methods. Colloids and Surfaces B: Biointerfaces, 2019, 174: 87-94. DOI: 10.1016/j.colsurfb.2018.10.080
Related Services
Support Documents
KNOWLEDGE CENTER
KNOWLEDGE CENTER












