- Services
- Demo
- FAQ
- Case Study
- Related Services
- Support Documents
- Inquiry
What is Extinction Coefficient?
The protein concentration is an important parameter affecting the efficacy of pharmaceutical products. Therefore, it is essential and necessary to determine the concentration of a protein solution during drug development. There are several methods for protein concentration determination, such as include UV absorption, the Braford assay, the bicinchoninic acid (BCA) assay, and so forth. To determine the protein concentration UV absorption, absorption in the UV region of the spectrum will be measure, and the concentration will be calculated according to the Beer-Lambert law, where extinction coefficient and path length are constant. Extinction coefficient, a measure of how strongly a substance absorbs light at a specific wavelength, is the intrinsic property of a protein depending on its composition and structure. Hence, to precisely determine protein concentration, it is fundamental to accurately determine extinction coefficient.
At Creative Proteomics, we provide expert extinction coefficient determination Services to support the accurate quantification and characterization of proteins. Our service adheres to industry standards, ensuring compliance with the ICH Q6B guidelines, and provides reliable results for protein concentration determination and related applications.
How is Extinction Coefficient Determined?
Absorbance Measurement: A sample is exposed to a monochromatic light source at the desired wavelength (typically 280 nm for proteins).
Concentration and Path Length: The concentration of the protein solution and the cuvette path length (usually 1 cm) are known.
Calculation: Using the Beer-Lambert equation (A/cL=ε), the extinction coefficient is calculated based on the absorbance values and known sample characteristics.
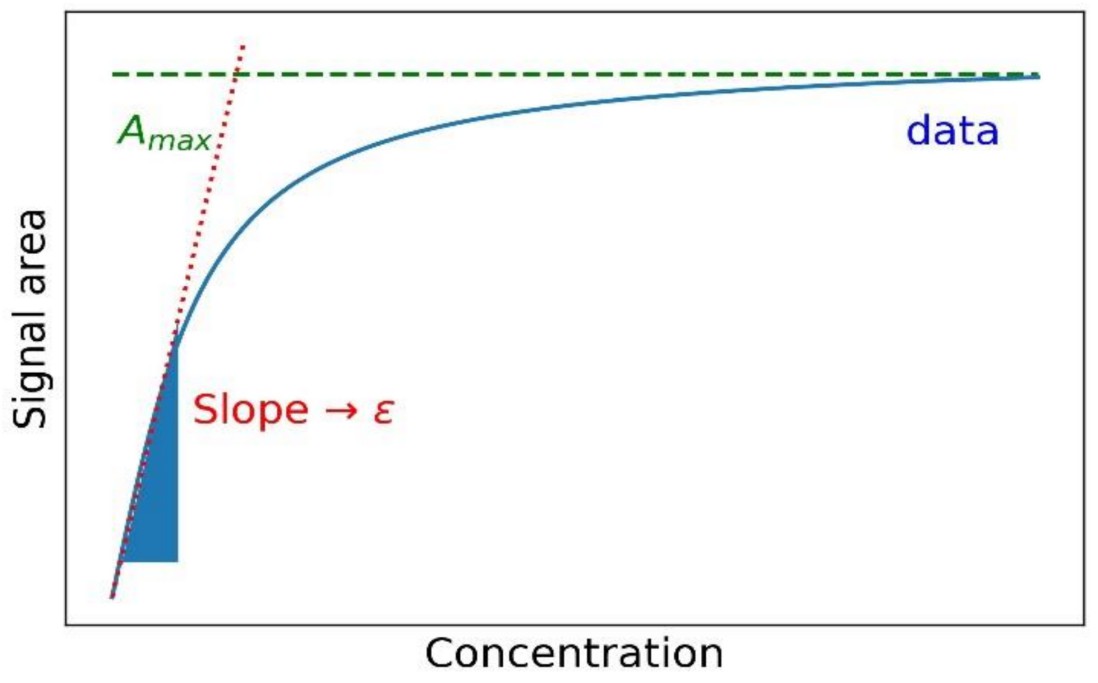 Figure 1. Determination of extinction coefficient. (Glorius M., 2020)
Figure 1. Determination of extinction coefficient. (Glorius M., 2020)We Can Provide (but not limited to):
- Accurate extinction coefficient determination for protein solutions
- Protein concentration analysis using UV-Vis spectrophotometry
- Purity assessment by analyzing the absorbance spectra of protein samples
Technology Platform of Extinction Coefficient Determination Service
The common approach to determine extinction coefficient is based on theoretical prediction from the amino acid sequence, but the spectral properties of a protein can be influenced by the folding state. Another approach to determine extinction coefficient take advantage of the photometric determination coupled with a measurement of absolute protein concentration, including total nitrogen determination, quantitative amino acid analysis, or dry weight determination. Here, a sedimentation velocity analytical ultracentrifugation (SV-AUC) based method equipped with absorbance and Rayleigh interference optics will be adopted to conveniently determine extinction coefficient.
Our extinction coefficient determination service mainly contains 3 steps:
1) Samples will be purified to minimize the effects of interfering buffer excipients.
2) Samples will be diluted, and the experimental AUC will be conducted with ProteomeLab XL-I equipped with interference and absorbance OPTICS.
3). AUC data will be analyzed.
One of advantages of SU-AUC based method is no limitations regarding column stability, etc. According to your special requirements, optimized techniques will be employed to accurately determine the extinction coefficient.

Advantages of Our Extinction Coefficient Determination Service
- Rich experience in protein analysis.
- High sensitivity and accuracy to detect proteins with different post-translational modifications.
- Batch-to-batch consistency.
- Customized service: according to you sample type and sample size, optimized protocols will be deployed.
Creative Proteomics's experienced scientists will provide you with a high-sensitivity and accurate extinction coefficient determination of biopharmaceutical products. Our service will be optimized to reach your special requirements. Clear and concise written reports including protocols and customized services will be offered during drug development.
Applications of Extinction Coefficient
- Protein Concentration Determination: The extinction coefficient allows for accurate protein concentration determination through absorbance measurements.
- Kinetic Studies: It enables the study of enzyme activity, protein-ligand binding, and other dynamic protein behaviors by tracking absorbance changes over time.
- Purity Assessment: By comparing expected extinction coefficients with absorbance measurements, it aids in assessing protein purity and identifying contaminants in a sample.
- Analytical Chemistry: Extinction coefficient determination is used in the development and validation of assays and protocols for protein analysis.
- Drug Development: In pharmaceutical research, determining the extinction coefficient is essential for the accurate quantification of therapeutic proteins and biologics.
Sample Requirements
| Sample Type | Minimum Volume | Concentration Range | Container Type |  Special Instructions |
|---|---|---|---|---|
| Protein Solution | 200 µL | 0.1 – 10 mg/mL | 1 cm cuvette (clear) | Protein sample should be free from contaminants like buffer salts or detergents. |
| Enzyme Solution | 200 µL | 0.1 – 5 mg/mL | 1 cm cuvette (clear) | Avoid the presence of interfering species like nucleic acids or lipids. |
| Purified Protein | 100 µL | 0.1 – 20 mg/mL | 1 cm cuvette (clear) | Samples should be at least 95% pure for accurate results. |
| Cell Lysate | 300 µL | 0.1 – 2 mg/mL | 1 cm cuvette (clear) | Should be clarified to remove insoluble particles. |
Demo
Demo: Protein Identification by Tandem Mass Spectrometry and Sequence Database Searching.
 Figure 2. An example of a tandem mass spectrometry spectrum.
Figure 2. An example of a tandem mass spectrometry spectrum.FAQ
Q: How is the quality of the sample assessed before starting the analysis?
A: We perform an initial visual inspection of the sample to check for clarity and any particulate matter. Additionally, a purity assessment is done using UV-Vis spectrophotometry to ensure that interfering substances, such as salts or contaminants, are minimized or removed, which could affect the accuracy of the extinction coefficient measurement.
Q: Can extinction coefficient determination be used to assess protein-ligand binding interactions?
A: Yes, the extinction coefficient can be part of studies on protein-ligand binding interactions. By monitoring changes in absorbance upon ligand binding, we can help determine the binding constants, identify conformational changes in the protein, and assess the nature of the interaction.
Q: How do you handle samples with multiple protein isoforms or aggregates?
A: When dealing with protein isoforms or aggregates, we work to isolate and quantify the distinct protein species, ensuring that the measurements accurately reflect the desired isoform. For aggregated proteins, we may suggest purification steps or employ methods to determine the extinction coefficient for individual species, as aggregates can skew absorbance values.
Q: Can extinction coefficient determination be used to study protein folding or conformational changes?
A: Yes, extinction coefficient determination can be used in studies of protein folding and conformational changes, particularly when coupled with techniques such as circular dichroism (CD) or fluorescence spectroscopy. Monitoring changes in absorbance due to folding or unfolding events can help deduce conformational stability and folding kinetics.
Case Study
Case: Chemical Characterization of Urate Hydroperoxide, A Pro-oxidant Intermediate Generated by Urate Oxidation in Inflammatory and Photoinduced Processes
Background
Urate hydroperoxide is a highly reactive oxidizing agent formed by the combination of the urate free radical and superoxide. While uric acid (urate) is traditionally considered an antioxidant, it can act as a pro-oxidant in certain environments, especially in inflammatory disorders and oxidative stress conditions. The formation of urate hydroperoxide, as an intermediate in urate oxidation, is hypothesized to contribute to oxidative damage in diseases like gout and other inflammatory conditions. This study aims to provide a detailed chemical characterization of urate hydroperoxide and investigate its reactivity with biomolecules.
Methods
- Electrochemical Analysis: The electrochemical behavior of urate hydroperoxide was assessed using cyclic voltammetry over a potential range from 1.0 to −1.0 V vs Ag/AgCl, analyzing the cathodic reduction of urate hydroperoxide.
- Reactivity Testing: The reactivity of urate hydroperoxide with various free amino acids (taurine, histidine, lysine, tryptophan, methionine, cysteine, and glutathione) was studied by incubating urate hydroperoxide with these amino acids and analyzing the products by HPLC and LC-MS/MS.
- Kinetic Studies: The kinetics of the reaction between urate hydroperoxide and glutathione were studied using pseudo-first-order conditions, and the rate constant was calculated.
- Spectroscopic Analysis: UV-Vis spectroscopy and HPLC were used to determine the molar extinction coefficient of urate hydroperoxide and its decomposition products.
Results
- Electrochemical Behavior: Urate hydroperoxide was reduced at around −0.5 V vs Ag/AgCl. The reduction product, hydroxyisourate, did not interfere with the electrochemical process.
- Reactivity with Amino Acids: Urate hydroperoxide selectively reacted with methionine, cysteine, and glutathione, forming oxidation products like methionine sulfoxide and cystine. No adducts were formed between urate hydroperoxide and these biomolecules, and the reaction followed a two-electron oxidation mechanism.
- Kinetics with Glutathione: The reaction of urate hydroperoxide with glutathione showed a second-order rate constant of 13.7±0.8 M⁻¹s⁻¹ at 23°C, confirming its high reactivity compared to other oxidants like hydrogen peroxide.
- Decomposition of Urate Hydroperoxide: Urate hydroperoxide decayed spontaneously with a half-life of 41 minutes at 22°C, forming hydroxyisourate and ultimately allantoin, a well-known end-product of urate oxidation. The molar extinction coefficient of urate hydroperoxide was found to be ε308nm=6.54±0.38×10³ M⁻¹cm⁻¹, allowing its concentration to be quantified accurately.
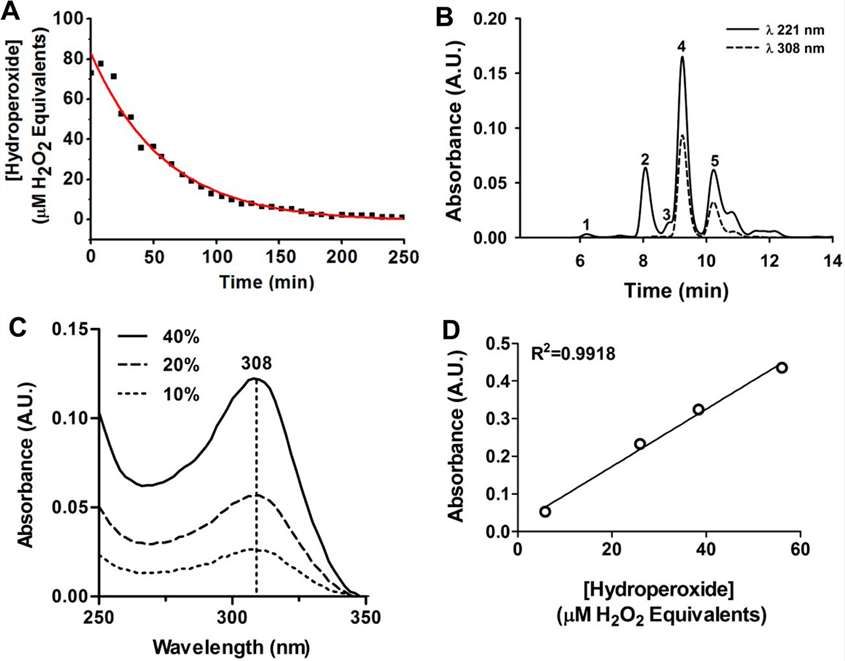 Figure 3. Kinetics of decomposition, products formation, and determination of the molar extinction coefficient (ε) of urate hydroperoxide.
Figure 3. Kinetics of decomposition, products formation, and determination of the molar extinction coefficient (ε) of urate hydroperoxide.References
- Glorius M, Reich T, Breitkopf C. Determination of Extinction Coefficients for Describing Gas Adsorption on Heterogeneous Catalysts Using In-Situ DRIFT Spectroscopy. Catalysts, 2020, 10(7): 735. DOI: 10.3390/catal10070735
- Patrício E S, et al. Chemical characterization of urate hydroperoxide, a pro-oxidant intermediate generated by urate oxidation in inflammatory and photoinduced processes. Chemical research in toxicology, 2015, 28(8): 1556-1566. DOI: 10.1021/acs.chemrestox.5b00132
- Hoffmann A., et al. Precise determination of protein extinction coefficients under native and denaturing conditions using SV-AUC. European Biophysics Journal, 2018, 47:761–768. DOI: 10.1007/s00249-018-1299-x
Related Services
Support Documents
KNOWLEDGE CENTER
KNOWLEDGE CENTER
KNOWLEDGE CENTER














