As Biotechnology and the pharmaceutical industry continue to develop, antibody-based therapeutics have become an integral part of the medical field. However, during the research and production of these antibody drugs, it's vital to ensure consistency and stability in their structure, thereby assuring their safety and effectiveness. Characterizing the proteins within these drugs and identifying their disulfide bond locations is a crucial step. In this context, mass spectrometry serves as a widely used analytical technique for protein characterization and disulfide bond analysis.
Amidst the ongoing progress in Biotechnology and the pharmaceutical sector, antibody-based therapeutics have emerged as a fundamental component of medical advancements. Ensuring structural consistency and stability is imperative in the research and production of these antibody drugs to guarantee their safety and efficacy. A pivotal step involves the characterization of proteins within these drugs and the identification of disulfide bond locations. In this regard, mass spectrometry stands as a prominently utilized analytical technique for comprehensive protein characterization and precise disulfide bond analysis.
How does mass spectrometry contribute to the development of antibody drugs?
Mass spectrometry, recognized for its exceptional sensitivity and resolution, is a sophisticated analytical technique widely acknowledged for its speed, accuracy, and reliability, particularly in the fields of life sciences and drug development. In the specific domain of antibody drug research, mass spectrometry plays an indispensable role, expediting a rapid and precise understanding of the structural nuances and quality attributes inherent to antibody drugs.
This powerful analytical tool allows for the in-depth analysis of the amino acid sequence of antibody medications. It facilitates the detection of various modifications and isomers existing in the protein, providing crucial insights into the molecular composition. Furthermore, mass spectrometry proves invaluable in pinpointing the position and quantity of disulfide bonds within the protein. This capability not only enables the assessment but also the optimization of the antibody drug's structure and stability. The acquired information assumes a pivotal role in guiding the intricate processes of research, development, and production of antibody drugs.
Mass spectrometry for the analysis of disulfide bonds
Mass spectrometry stands out as a promising avenue for protein characterization, particularly in its crucial role in the analysis of disulfide bonds. Traditional approaches to disulfide bond analysis frequently rely on reduction-alkylation or enzymatic cleavage, each fraught with inherent limitations, including intricate sample processing, labor-intensive procedures, and diminished sensitivity.
However, leveraging mass spectrometry for determining the location and count of disulfide bonds in proteins not only enhances the accuracy and precision of analysis but also facilitates high throughput and high sensitivity analyses.
In terms of disulfide bond analysis, there are two main types of mass spectrometry techniques: isotopic labeling techniques and topological analysis techniques. Isotopic labeling techniques usually employ Cysteine-reactive isotopic tags (CRIT) to mark cysteine, and they identify the locations and quantities of disulfide bonds by comparing mass spectrometry data before and after labeling. Topological analysis techniques correspond the geometric structure of a protein molecule to its mass peak shape, thereby conjecturing the position of the disulfide bond. Both techniques greatly facilitate research and development of antibody drugs.
Select Service
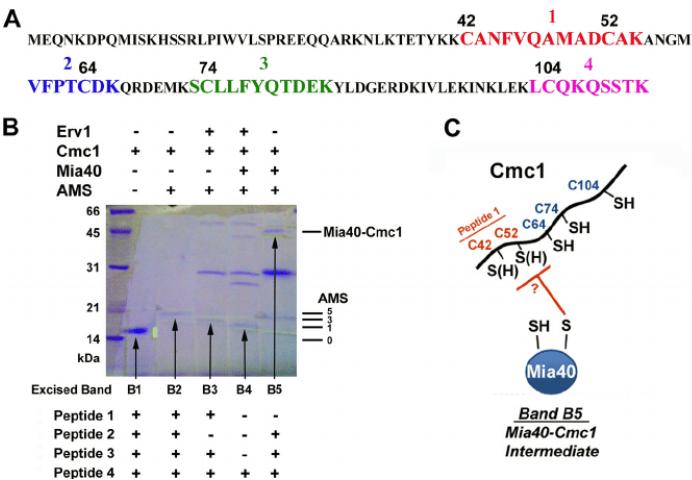 Identification of disulfide bonds by mass spectrometry. (Myriam Bourens et al,. 2012)
Identification of disulfide bonds by mass spectrometry. (Myriam Bourens et al,. 2012)Other applications of mass spectrometry in antibody drug discovery and development
In addition to disulfide bond analysis, mass spectrometry also finds diverse applications in the research and development of antibody drugs. For instance, this technique can be harnessed to analyze glycosylation of antibody drugs, determining the type and location of the attached sugar groups. This, in turn, enables the assessment and optimization of the drugs' stability and immunogenicity. Equally, mass spectrometry can be used to scrutinize conformational changes and aggregation phenomena within the antibody drugs, as well as facilitating the study of their pharmacokinetics.
Disulfide bonds and molecular dynamics
Mass spectrometry, as a high-sensitivity, high-resolution novel analytical method, presents broad application prospects in the development of antibody drugs. In particular, its role in protein characterization and disulfide bond analysis has established it as one of the most promising techniques. We believe that, in the context of ongoing advancements and innovations in science and technology, mass spectrometry techniques will become increasingly sophisticated and refined, fostering a plethora of possibilities and opportunities for the development of antibody drugs and other fields of study.
Therefore, for researchers engaged in the development of antibody drugs, it is essential to gain a deep understanding and mastery of relevant knowledge and skills related to mass spectrometry. By actively implementing mass spectrometry for the structural characterization and disulfide bond analysis of antibody drugs, the advancement of antibody drug development can be expedited. Moving forward, mass spectrometry will continue to play an integral role in the development of antibody drugs, contributing significantly to the improvement of human health.
Furthermore, beyond the utilization of mass spectrometry, antibody drug development has witnessed the integration of various innovative analytical methods and technologies in recent years. Optical technology, nuclear magnetic resonance technology, and electrophoresis technology, among others, play instrumental roles in the structural and functional characterization of antibody drugs. These approaches contribute to furnishing more comprehensive and precise insights essential for the advancement and production of these therapeutic entities.
Particularly for disulfide bonds, Molecular Dynamics Simulation, another widely applied technique in antibody drug R&D, aside from MS. MD Simulation allows the simulation of the process of formation and breaking of disulfide bonds in proteins at the atomic level, thereby predicting the position and number of disulfide links, and evaluating the stability and reliability of antibody drugs. This technique, recognised for its efficiency, accuracy, and cost-effectiveness, provides essential support for antibody drug development.
In summary, the relentless advancement and innovation in science and technology have led to the incorporation of an expanding array of cutting-edge techniques and methodologies in the exploration and development of antibody drugs. These diverse methods not only enhance our comprehension of the structural features and functions of antibody drugs but also yield a more exhaustive and accurate dataset crucial for guiding the research, development, and production phases. We harbor confidence that, in the foreseeable future, the continuous evolution and refinement of these techniques and methodologies will make substantial contributions to the enhancement of public health.









