Introduction
Nucleic acid therapeutics, as the name suggests, are designed around nucleic acid molecules to directly intervene in genetic material transcription and regulatory processes for therapeutic purposes. In recent years, nucleic acid therapeutics have emerged as the third generation of innovative drugs following small molecules and antibody therapeutics, marking the forefront of biomedical development. Depending on their mechanisms of action and application domains, nucleic acid therapeutics can be categorized into oligonucleotide drugs, small molecule nucleoside drugs, mRNA vaccines, and others.
What are Therapeutic Oligonucleotide
Oligonucleotide drugs refer to nucleic acid sequences of less than 30 nucleotides, including small interfering RNA (siRNA), microRNA (miRNA), antisense oligonucleotides (ASO), and nucleic acid aptamers. These drugs exert their therapeutic effects by binding to target RNA molecules, thereby inhibiting their translation or regulation. Oligonucleotide drugs exhibit high target specificity and selectivity, enabling precise modulation of gene expression. Consequently, they hold promising applications in gene therapy and gene editing fields.
Table 1. FDA-Approved Oligonucleotide Therapeutics for Rare Diseases
| OGN | Organization | Therapeutic Type | Target |
| Vitravene | Novartis | Antisense | Cytomegalovirus retinitis |
| Kynamro | Sanofi Genzyme | Antisense | Hypercholesterolemia |
| Eteplirsen | Sarepta Therapeutics | Splice Switching | Duchenne muscular dystrophy |
| Nusinersen | Biogen | Splice Switching | Spinal muscular atrophy |
| Tegsedi | Akcea Therapeutics | Antisense | Hereditary transthyretin-mediated amyloidosis |
| Vyondys 53 | Sarepta Therapeutics | Splice Switching | Duchenne muscular dystrophy |
| Onpattro | Alnylam Pharmaceuticals | siRNA | Hereditary transthyretin-mediated amyloidosis |
| Givlaari | Alnylam Pharmaceuticals | siRNA | Acute hepatic porphyria |
| Pegaptanib | Eyetech Pharmaceuticals | Aptamer | Age-related macular degeneration |
| Defitelio | Jazz Pharmaceuticals | Stimulatory | Prevention of blood clotting |
Oligonucleotide Synthesis and Modification
The synthesis of oligonucleotides predominantly employs automated instruments utilizing solid-phase phosphoramidite chemistry. However, achieving 100% efficiency at each synthetic step is inherently unattainable. Consequently, within the full-length oligonucleotide sequences, impurities such as n-1 and n+1 variants persist, exhibiting structural and sequential similarities to the desired therapeutic molecule. Unmodified oligonucleotide drugs are susceptible to rapid degradation by nucleases in vivo, compromising their pharmacological efficacy. Thus, to optimize clinical outcomes, oligonucleotides are often subject to chemical modifications and enhancements. Common strategies include the introduction of phosphorothioate linkages to fortify against nuclease degradation, and 2'-O-Me modifications to enhance target affinity. The extensive modification of oligonucleotides poses significant analytical challenges, necessitating chromatographic analysis for purification, impurity characterization, and assessment of structural variants. Employing multiple chromatographic techniques is often imperative to surmount challenges such as peak broadening and low resolution inherent in single-method analyses.
You may interested in
Oligonucleotides Analytical Method
Reversed-Phase Chromatography
For oligonucleotide drugs, Ion-Pair Reversed-Phase Chromatography (IP-RP) stands as the preferred method for purity analysis. Given the substantial molecular weight of oligonucleotide drugs and their carriage of multiple negative charges, their pronounced hydrophilicity necessitates the utilization of ion-pair reagents such as triethylamine to enhance retention on the reversed-phase column. Concurrent use of hexafluoroisopropanol (HFIP) further facilitates mass spectrometric compatibility.
During IP-RP analysis, various factors including the type, concentration, column temperature, and mobile phase pH of ion-pair reagents (IPRs) significantly influence separation efficiency. Typically, two classes of classical ion-pair reagents are employed based on the charge properties of the analyte:
Quaternary ammonium salts: Positively charged, utilized to attract anionic analytes, their inclusion in the mobile phase enhances retention of anionic species such as triethylamine and tetrabutylammonium bromide.
Alkyl sulfonate salts: Negatively charged, capable of complexing with cationic analytes, their addition to the mobile phase enhances retention of cationic species, for instance, sodium dodecyl sulfate and sodium octyl sulfate.
When coupled with mass spectrometry, it is customary to reduce the concentration of ion-pair reagents in the mobile phase to mitigate the deleterious effects of high IPR concentrations on ionization efficiency. Additionally, the incorporation of HFIP into the mobile phase enhances ionization efficiency by augmenting the surface charge during Electrospray Ionization (ESI). The simultaneous use of triethylamine and HFIP in the mobile phase has been demonstrated to effectively discern a spectrum of oligonucleotide impurities, including n-1, n+1, and P=O variants.
Mass Spectrometry (MS)
High-resolution Liquid Chromatography-Mass Spectrometry (LC-MS), Size Exclusion Chromatography (SEC), and Capillary Gel Electrophoresis (CGE) are employed for the analysis of molecular mass, size, and folding of oligonucleotides. These attributes are identified based on retention time or molecular weight. Differences in retention time between different batches can indicate structural or conformational changes.
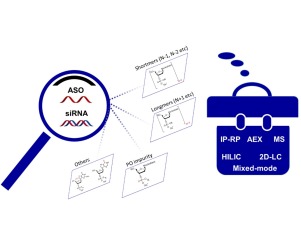 Characterization of therapeutic oligonucleotides by liquid chromatography
Characterization of therapeutic oligonucleotides by liquid chromatographyLC-MS in Tandem with CGE or LC
The tandem use of MS with CGE or LC enables the identification of potential mass differences among smaller lengths (<100nt). Electrospray Ionization Mass Spectrometry (ESI-MS), a technique combining chromatographic separation with mass spectrometry, preserves the structure of oligonucleotides through soft ionization techniques, followed by collision-induced dissociation, generating predictable fragment ions. Increased chemical modification of oligonucleotides complicates the fragmentation ions produced by mass spectrometry, thereby increasing sequencing challenges.
Capillary Gel Electrophoresis (CGE)
CGE serves as a quantitative method used for purity analysis, enabling the analysis of oligonucleotide size and folding. Prior to analysis, it requires denaturation of oligonucleotides by adding denaturing agents to the analysis buffer. When coupled with mass spectrometry, CGE faces challenges such as buffer effects, sampling biases, and challenges related to cationic intramolecular interactions.
Size Exclusion Chromatography (SEC)
SEC is also utilized for analyzing the size of oligonucleotides. Conformational characterization is particularly important for aptamers as it directly correlates with ligand binding. SEC can also study the polydispersity of drug delivery conjugates by analyzing the hydrodynamic radius of the molecules. However, SEC methods encounter challenges of low resolution in oligonucleotide analysis. Chromatographic methods must optimize the pore size of the stationary phase based on the size of the analyte to achieve optimal separation.
Anion Exchange Chromatography (AEX)
AEX analyzes differences in the number and position of negatively charged groups on the surface of the analyte. Given the presence of negatively charged phosphate groups on the oligonucleotide drug backbone, AEX is commonly used for purity and impurity analysis of oligonucleotides. Furthermore, AEX can to some extent achieve stereoisomeric resolution of oligonucleotides modified with phosphorothioate linkages, providing a basis for the characterization of their stereochemistry. The main drawback of AEX is its incompatibility with mass spectrometry.
Table 2. Liquid Chromatography Columns and Applicable Conditions for the Analysis of Oligonucleotide Drugs
| Chromatography Column | Particle Size (μm) | Stationary Phase | Maximum Pressure (bar) | Pore Size (A) | pH Range | Maximum Temperature (℃) | Recommended Mobile Phase | |
| IP-RP | ||||||||
| Clarity Oligo RP (Phenomenex) | 3-10 | Porous | 345 | 110 | 1-12 | 60 | 50 mM TEAA,pH 7.5, 5%v/v Me CN +Me OH or 15 mM DMCHA:25mM HFIP +MeOH | |
| Advance Bio C18 (Agilent) | 2.7 | Core shell | 600 | 100 | 3-11 | 65 | 100 mM TEA A+Me CN or 400:15 mM HFIP:TEA+MeOH | |
| Accucore C18 (Thermo Fisher) | 2.6 | Core shell | 1000 | 80-150 | 1-11 | 70 | ||
| DNAPac RP (Thermo Fisher) | 4 | Polymeric | 275 | NA | 0-14 | 110 | ||
| Acquity UPLC BEH C18 (Waters) | 1.7 | Hybrid | 1241 | 130 | 1-12 | 90 | ||
| Xbridge BEH Phenyl (Waters) | 2.5 | Hybrid | 400 | 130 | 1-12 | 60 | 100 mM TEA A or 5 mM TBuAA+MeCN | |
| Xbridge (Waters) | BEH Amide | 2.5 | Hybrid | 400 | 130 | 2-11 | 90 | |
| SEC | ||||||||
| Advance Bio SEC (Agilent) | 1.9-2.7 | Porous | 400-600 | 130-300 | 2-8.5 | 60 | 150 mM sodium phosphate buffer,pH 7 | |
| Zenix SEC (Seepax) | 3 | 310 | 150-300 | 2-8.5 | 80 | |||
| Shimpac Bio Dio (Shimadzu) | 2-5 | 200-450 | 60-300 | 5-7.5 | 50 | 100 mM sodium phosphate buffer+2 M NaCl,pH 7 | ||
| EC | ||||||||
| PL-SAX(Agilent) | 8 | Core shell | 207 | 1000 | 1-14 | 60 | 7:93 v/v MeCN:0.1 M TEAA,1 M CIH₄N, pH 8.5 | |
| Shimpac Bio IEX (Shimadzu) | 3-5 | Polymeric | 250-350 | NA | 2-12 | 60 | 10 mM NaOH,1 M NaClO₄ | |
| Clarity Oligo-SAX (Phenomenex) | 5 | 345 | NA | 2.5-12.4 | 85 | 20mM Tris,1.25 M NaCl or 1 M NaClO₄, pH 8 | ||
| DNAPac PA200 Rs (Thermo Fisher) | 4 | 689 | NA | 4-12.4 | 85 | |||
| HILIC | ||||||||
| Luna HILIC (Phenomenex | 3-5 | Porous | 345 | 200 | 1.5-8 | 60 | 5 mM C₂H₂NO₂ in H₂O, pH 5.8:MeCN | |
| HALO HILIC (AMT) | 2-5 | Core shell | 620 | 90 | 1-8 | 60 | 90:10 v/v MeCN:0.1 M CHsNO₂ | |
Therapeutic Oligonucleotides Research Trends
The analytical strategies employed to characterize oligonucleotide drugs for the determination of critical attributes such as sequence, conformation, and structure are multifaceted. Therapeutic oligonucleotides, as an emerging therapeutic modality, continue to have their potential explored, spearheading a global wave of research and development. In the forthcoming years, further advancements in analytical methods hold promise in overcoming current challenges, thereby enhancing the reliability of quality analysis and control of oligonucleotide drugs.
References
- J.V. Bonilla and G.S. Srivatsa, Handbook of Analysis of Oligonucleotides and Related Products (CRC Press, Boca Raton, Florida, 2011).
- D. Capaldi, K. Ackley, D. Brooks, J. Carmody, K.Draper, R. Kambhampati, M. Kretschmer, D. Levin,J. McArdle, B. Noll, R. Raghavachari, I. Roymoulik, B.P. Sharma, R. Thurmer, and F. Wincott, Ther.Innov. Regul. Sci. 46(5), 611–626 (2012).
- N. Elzahar, N. Magdy, A. El-Kosasy, and M. Bartlett,Anal. Bioanal. Chem. 410(14), 3375–3384 (2018).
- Goyon A, Yehl P, Zhang K. Characterization of therapeutic oligonucleotides by liquid chromatography. J Pharm Biomed Anal. 2020







