Classification of Protein Glycosylation
Protein glycosylation is a critical post-translational modification that significantly impacts protein structure and function. This process primarily involves two types of glycosylation modifications: N-glycosylation and O-glycosylation.
N-Glycosylation
N-glycosylation occurs at a characteristic sequence within the protein's primary structure, denoted as NXT, where X represents any amino acid except proline. This modification involves the attachment of glycans to the nitrogen atom of asparagine side chains.
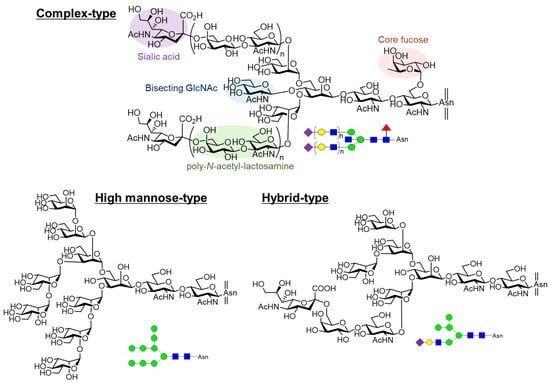 Figure 1. Structures of N-glycans.
Figure 1. Structures of N-glycans.N-Glycan Biosynthesis
The initial N-glycan modification begins in the endoplasmic reticulum (ER), where a glycan moiety composed of Glc3Man9GlcNAc2 units is transferred to the side chain amino group of asparagine residues. Subsequently, these glycans undergo processing within the ER and Golgi apparatus, resulting in the formation of three main types of N-glycans:
High-mannose type: Characterized by a high mannose content.
Complex type: Features a more intricate sugar structure.
Hybrid type: Contains elements of both high-mannose and complex types.
O-Glycosylation
O-glycosylation occurs on serine (S) and threonine (T) residues of proteins, where the attached sugar moiety connects to the hydroxyl group of these amino acids. The biosynthesis of O-glycans follows the N-glycosylation modification, protein folding, and assembly phases. The process involves various monosaccharide structures, with the most common being:
N-acetylgalactosamine (GalNAc)
N-acetylglucosamine (GlcNAc)
Xylose
Mannose
Fucose
These monosaccharides extend from the core GalNAc, forming mucin-like O-glycan structures.
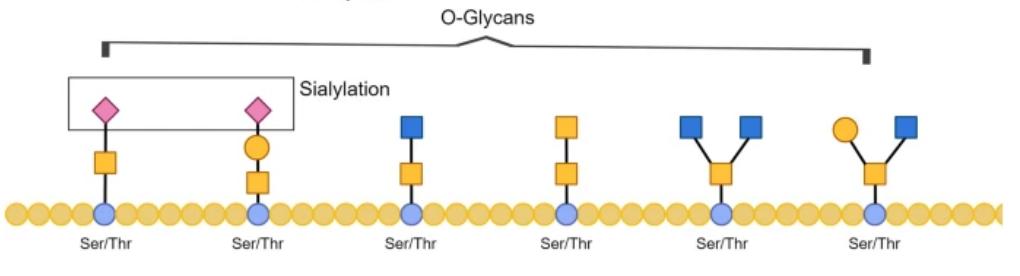 Figure 2. The O-GlcNAc is a single GlcNAc being added to the Ser/Thr in the polypeptide chain.
Figure 2. The O-GlcNAc is a single GlcNAc being added to the Ser/Thr in the polypeptide chain.Variability in Glycosylation Patterns
Not all N-glycan modifications occur at every characteristic sequence, and not all serine/threonine residues are subject to O-glycosylation. This variability leads to heterogeneity in glycosylation sites. Additionally, competition among different enzymes during biosynthesis results in micro-heterogeneity at each glycosylation site, where distinct glycan structures can be present.
Synthesis Pathway of N-Glycans
The synthesis of N-linked glycans is a complex process involving multiple stages and enzymatic reactions. The initial donor for N-glycan synthesis is a Glc3Man9GlcNAc2 structure, which attaches to a lipid dolichol via a pyrophosphate bond. Dolichol, embedded within the lipid bilayer, adopts either a helical or folded conformation. The assembly of the glycan on dolichol proceeds in two distinct phases:
Glycan Assembly Stages
Stage One: Cytoplasmic Side of the Endoplasmic Reticulum
The first phase occurs on the cytoplasmic side of the endoplasmic reticulum (ER) membrane. During this stage, enzymes catalyze the attachment of two GlcNAc residues and five mannose residues to the growing glycan chain. This reaction utilizes nucleotide donors uridine diphosphate (UDP)-GlcNAc and GDP-mannose. The lipid-linked glycan is subsequently translocated across the ER membrane. As the glycan chain grows, it is exposed to the luminal side of the ER membrane, where additional sugars are added. The final four mannose residues and three glucose residues are contributed by the glycan donor connected to dolichol. These sugars are synthesized via reactions between dolichol phosphate and UDP-Glc or GDP-mannose on the cytoplasmic surface of the ER membrane. Oligosaccharides are then transferred from dolichol to the nascent protein's N-glycosylation site, specifically the asparagine residue, with the assistance of oligosaccharyltransferase, concomitant with the protein's proper folding.
Stage Two: Golgi Apparatus Processing
Following the transfer to the Golgi apparatus, a series of specific enzymes are responsible for trimming monosaccharides from the glycan chain's terminal ends. This includes α-glucosidase I and α-mannosidase I/II. Additionally, various enzymes add nucleotide-sugars to the glycan chain, such as N-acetylglucosamine transferase I/III, fucosyltransferase, galactosyltransferase, and sialyltransferase. The dynamic modifications during glycan synthesis result in different N-glycan types, leading to high heterogeneity in N-glycans.
Significance of N-Glycan Biosynthesis
Understanding the biosynthesis pathway of N-glycans is crucial for comprehending the quality attributes of therapeutic glycoproteins. It also aids in modifying expression cells to alter the biological properties of therapeutic proteins. Variations in cell growth conditions and production processes result in different glycan profiles, which can serve as sensitive indicators of production process stability. Manufacturers should conduct N-glycan testing to ensure product consistency.
For instance, Roche has addressed potential Fc-region effects of Atezolizumab by removing M-glycans. This strategy involves mutating asparagine residues at glycosylation sites on the Fc region to other amino acids. Additionally, enhancing antibody-dependent cellular cytotoxicity (ADCC) can be achieved by knocking out fucosyltransferase or overexpressing N-acetylglucosamine transferase III in expression cells. This adjustment reduces the core fucose content. Reports suggest that adding magnesium ions or butyrate to the culture medium can increase terminal galactose content on antibodies, thereby enhancing complement-dependent cytotoxicity (CDC) functions.
N-Glycan Nomenclature
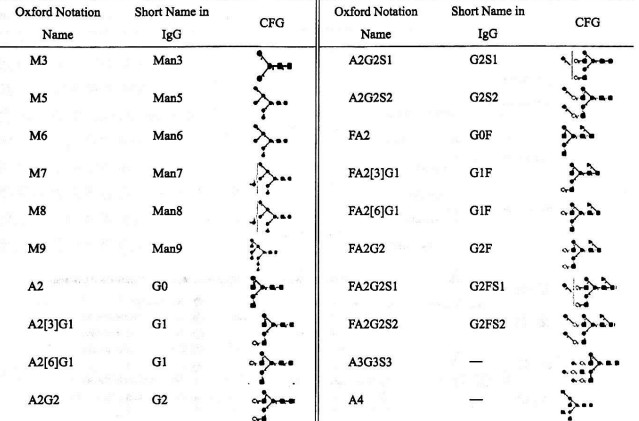
The complexity of N-glycans presents challenges in accurately describing their structures. A comprehensive description of N-glycans encompasses three main aspects: the types of monosaccharides, the linkage positions between monosaccharides, and the linkage conformations. The International Union of Pure and Applied Chemistry (IUPAC) has proposed and standardized the most precise method for describing N-glycans. This method describes monosaccharide types, linkage conformations, and positions in detail, providing accurate descriptions but being relatively complex in writing and comprehension.
Simplified Nomenclature Methods
To simplify the description of N-glycan structures, two alternative nomenclature systems have been developed: the Consortium for Functional Glycomics (CFG) and the Oxford nomenclature. These systems were proposed by the Functional Glycomics Consortium Nomenclature Committee and the Glycobiology Department at Oxford University, respectively, and are now widely used in academic communication among glycobiologists. These methods facilitate a more accessible representation of glycan structures.
Due to the relatively fixed patterns of monosaccharide linkages and positions on human protein N-glycans, descriptions may sometimes omit details on linkage positions and conformations. This simplified approach is commonly employed when describing N-glycan types in therapeutic glycoproteins.
Simplified Nomenclature for Monoclonal Antibodies
For monoclonal antibodies, the majority of N-glycan modifications occur predominantly at the asparagine residue located around position 297 on the heavy chain, within the CH2 domain. The glycan structures at this site are relatively simple, as most antibodies are expressed in mammalian cell lines such as CHO, SP20, and NS0. These expression systems tend to produce glycan structures with fixed linkage conformations and positions, allowing for simplified textual descriptions.
Table 1 Comparative of Nomenclature Systems
The following table correlates the simplified description format with the Oxford textual description format and the CFG structural representation:
The Significance of N-Glycan Quality Control in Recombinant Protein Therapeutics
Impact of N-Glycans on Protein Efficacy
N-glycans play a pivotal role in determining the pharmacokinetic properties of recombinant proteins. For example, erythropoietin (EPO), employed in the treatment of anemia, encompasses three N-glycans and one O-glycan. The sialic acid residues at the non-reducing termini of these N-glycans are instrumental in modulating the in vivo half-life of EPO. Notably, mutations introducing two additional N-glycans at five specific amino acid residues have been demonstrated to significantly prolong the half-life of EPO. Similarly, tissue-type plasminogen activator (TPA) contains high-mannose N-glycans within its kringle and EGF domains, which influence its clearance rate. Alterations in these glycan structures via site-specific mutations result in an enhanced half-life, which may decrease the required dosage or frequency of administration.
Effect on Antibody-Dependent Cellular Cytotoxicity (ADCC)
The N-glycans located in the Fc region of monoclonal antibodies (mAbs) are critical for their biological activity, particularly their capability to mediate ADCC. It has been observed that the presence of fucose within the Fc region's N-glycans inversely correlates with ADCC activity. Specifically, a 1% increase in afucosylation can result in a marked enhancement of ADCC activity, underscoring the imperative role of glycan composition in modulating therapeutic efficacy.
Influence on Immunogenicity
N-glycans also modulate the immunogenicity of recombinant proteins. Cetuximab, produced in murine SP20 cells, contains terminal α-galactose on its N-glycans. This glycan structure can interact with pre-existing anti-α-galactose antibodies in humans, potentially leading to severe allergic reactions. This phenomenon underscores the necessity of excluding non-human glycan epitopes in recombinant proteins to mitigate immunogenic responses.
Production Process and Glycan Variability
The glycosylation pattern of recombinant proteins is highly sensitive to variations in the production process. Factors such as the concentration of magnesium ions and butyrate in the culture medium can significantly alter the levels of G1F and G2F glycan structures on mAbs. These alterations can affect their complement-dependent cytotoxicity (CDC) function. Ensuring the stability and reproducibility of the production process is crucial for maintaining consistent glycan profiles before and after process modifications.
Biosimilar Development
The development of biosimilars necessitates comprehensive quality studies of multiple batches of the reference product, with a particular focus on glycan profiles. The production process is then meticulously optimized to achieve glycan profiles in biosimilars that closely replicate those of the reference product. This optimization is critical for ensuring that the efficacy and safety profiles of the biosimilars are comparable to those of the original therapeutic.
N-glycan quality control is essential for optimizing the therapeutic efficacy and safety of recombinant proteins. Detailed analysis and strict regulation of glycosylation patterns during production can significantly improve pharmacokinetics, minimize immunogenic responses, and ensure consistent therapeutic performance. This meticulous approach is particularly crucial in the development of biosimilars, where maintaining glycan profile parity with the reference product is paramount.
Impact of N-Glycan Structures on Monoclonal Antibody Structure and Function
N-glycans significantly influence the structural integrity and functional efficacy of mAbs. This article provides a detailed examination of how N-glycan structures affect mAb properties, including their conformation, antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), half-life, immunogenicity, and anti-inflammatory effects.
Structural Integrity of Monoclonal Antibodies
Monoclonal antibodies are immunoglobulins consisting of two Fab regions and one Fc region. The Fab regions bind specific antigens, while the Fc region mediates effector functions such as ADCC and CDC. In serum immunoglobulins, the Fc region contains a conserved N-glycosylation site within the CH2 domain, with approximately 30% of antibodies also having an N-glycosylation site in the Fab region. Serum antibodies exhibit complex bisecting glycan structures, with some featuring branched N-acetylglucosamine.
In contrast, recombinant mAbs, predominantly expressed in CHO, SP2/0, and NS0 cells, typically exhibit N-glycosylation exclusively on the heavy chain Fc region (with the exception of cetuximab) and lack branched N-acetylglucosamine structures. The N-glycan structure is crucial for maintaining the conformation and functionality of mAbs.
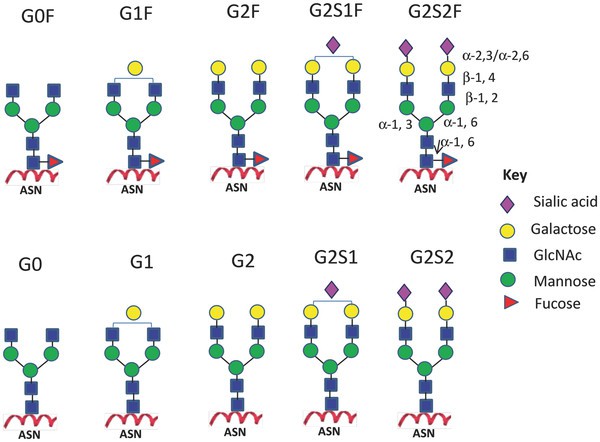 Figure 3. Major N-linked glycoforms of mAb therapeutics.
Figure 3. Major N-linked glycoforms of mAb therapeutics.Maintenance of Conformation
The G0 glycan structure on mAbs facilitates 52 hydrophobic interactions within the FcCY2 domain. Core fucose contributes seven interactions, while galactose on the α1-6 arm accounts for 27 interactions. Notably, glycans on the α1-3 arm do not interact with the antibody surface but penetrate the spatial region formed by the two heavy chain Fc domains. The interaction between mannoses on the α1-3 arm is critical for maintaining mAb conformation. Absence of glycan chains results in slight expansion of the Fc CH2 domain, manifesting as an earlier elution time in size exclusion chromatography and increased sensitivity to aggregation in thermal stability assays. This loss of glycan integrity leads to diminished ADCC and CDC activities and heightened protease sensitivity.
Relationship with ADCC
N-glycans on the Fc region can contain core fucose linked in an α1-6 manner to core N-acetylglucosamine. The absence of core fucose enhances the binding affinity between the Fc region and Fcγ receptors (FcR), thereby significantly increasing ADCC efficacy. The enzyme α1-6 fucosyltransferase (FUT8) is responsible for transferring fucose to core N-acetylglucosamine. Knockout of FUT8 in engineering cells produces mAbs devoid of core fucose, as exemplified by the POTeLlIGeNT technology, which has successfully advanced multiple mAbs into clinical trials.
Additionally, branched N-acetylglucosamine can obstruct core fucose generation. Overexpression of β1-4 N-acetylglucosamine transferase in engineered cells, leading to increased synthesis of branched N-acetylglucosamine, enhances ADCC. Roche's Glycart technology exemplifies this approach, with the marketed product Obinutuzumab (GA101) demonstrating improved ADCC. High-mannose N-glycans can also enhance ADCC, potentially due to the lack of core fucose. Sialylation of terminal glycans on the Fc region may reduce ADCC efficacy, as high sialylation can weaken Fc interactions with Fcγ receptors and alter Fab valency, affecting membrane antigen binding.
Relationship with CDC
The lactose moiety at the terminal N-glycan of rituximab's Fc region correlates with its CDC activity. Removal of terminal galactose results in decreased CDC efficacy. Studies emphasize the importance of monitoring terminal galactose levels on mAb Fc N-glycans. Gramer et al. demonstrated that adjusting media components such as purine, manganese chloride, and galactose in fed-batch fermentation of GS-CHO cells significantly elevates terminal galactose levels on mAbs. Conversely, mAbs with high-mannose N-glycans exhibit reduced CDC activity, likely due to the lack of terminal galactose. However, the relationship between terminal galactose levels and CDC efficacy in other mAbs warrants further investigation.
Relationship with Half-Life
The prolonged half-life of antibodies is primarily due to their interaction with neonatal Fc receptors (FcRn) expressed on endothelial cells and macrophages. FcRn binds circulating antibodies and facilitates their release into the bloodstream, protecting them from degradation. Non-sialylated glycoprotein receptors bind terminal galactose and N-acetylglucosamine, mediating degradation through endocytosis and reducing protein half-life. For instance, erythropoietin with high sialylation avoids terminal galactose exposure, extending its half-life. Different glycosylation types on Fc regions do not appear to affect mAb half-life significantly, possibly due to spatial hindrance preventing interaction with asialoglycoprotein receptors (ASGPR). Although mannose receptors can bind terminal mannose residues similar to ASGPR, some studies suggest that high-mannose N-glycans may reduce mAb levels rather than shorten their half-life. The consensus indicates that high-mannose N-glycans are associated with a reduced half-life, necessitating careful control of their proportion in mAbs.
Relationship with Immunogenicity
Glycoproteins expressed in rodent cell lines contain non-human sugars, such as α-galactose (Gal) and N-glycolylneuraminic acid (Neu5Gc). Chinese hamster ovary (CHO) cells express minimal Neu5Gc, whereas mouse myeloma cell lines (including SP2/0 and NS0) typically exhibit higher Neu5Gc levels. Humans lack the gene for α-galactose synthesis and possess mutated genes for Neu5Gc synthesis, leading to antibodies against α-galactose and Neu5Gc. This can result in immune responses against mAbs containing these non-human sugars. Approximately 40% of marketed mAbs are expressed in SP2/0 or NS0 cells, which may induce immune reactions due to the presence of α-galactose and Neu5Gc. Cetuximab, expressed in SP2/0 cells, contains α-galactose in the Fab region, causing severe allergic reactions and reduced half-life. However, most mAbs, including those from Fc N-glycosylation sites, do not bind to pre-existing anti-α-galactose antibodies. Our research indicates that only mAbs with two or more Neu5Gc residues on the Fc region can bind anti-Neu5Gc antibodies, suggesting minimal immunogenicity.
High Sialylation and Anti-Inflammatory Effects
Kaneko et al. demonstrated that high sialylation components of intravenous immunoglobulin (IVIG) possess anti-inflammatory properties. Anthony et al. further confirmed that the active component is high-sialylation N-glycans. Removal of sialic acid from IVIG abolishes its anti-inflammatory effect. High sialylation enhances the tightness of the Fc structure, reducing its binding to Fc receptors and increasing binding to the immune checkpoint receptor DC-SIGN, thereby exerting anti-inflammatory effects. This opens avenues for mAbs to replace IVIG in anti-inflammatory treatments. Notably, sialic acid in IVIG is predominantly α2-6 linked, whereas CHO cell-expressed mAbs typically contain α2-3 linked sialic acid. GlycoFi technology, developed by Hamilton et al., involves yeast genetic modifications to produce antibodies with high sialylation, demonstrating potential for therapeutic applications.
References
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22.
- Shirakawa, A.; Manabe, Y.; Fukase, K. Recent Advances in the Chemical Biology of N-Glycans. Molecules 2021, 26, 1040.









