Antibody aggregates constitute stable complexes formed through intermolecular forces, including covalent bonds and hydrogen bonds, between antibody molecules. These aggregates, comprising two or more protein molecules, result from intricate processes involving structural and conformational alterations in the protein. The presence of antibody aggregates can profoundly affect the efficacy and safety of therapeutic antibodies. This manuscript investigates the mechanisms responsible for the formation of antibody aggregates, their regulatory implications, and strategies to mitigate their presence.
What is an Antibody Aggregate
Antibody aggregates represent polymeric complexes formed through intermolecular interactions, such as covalent and hydrogen bonds, between antibody molecules. The aggregation process is intricate, involving various structural and conformational alterations in the protein.
Mechanisms Underlying Antibody Aggregation
Protein Structural Disruption: Structural integrity deficits in proteins can lead to the formation of aggregates. These deficits often arise from denaturation processes or other conformational disruptions, prompting the aggregation into polymeric structures.
Conformational Changes: Shifts in the spatial configuration of proteins can induce the formation of irreversible aggregates. Environmental factors, including pH fluctuations or temperature variations, destabilize the native protein structure, thereby facilitating aggregation.
Dimerization and Aggregate Growth: Aggregation frequently initiates with the association of two protein molecules, forming a dimer. Subsequent monomer addition results in the progressive increase in aggregate size.
Misfolding of Fab and Fc Domains: Incorrect folding of the Fab (antigen-binding) and Fc (constant) regions of the monoclonal antibody can promote aggregate formation. Misfolded or unfolded regions enhance the propensity for protein molecules to associate into larger aggregates.
Disulfide Bond Formation: Disulfide bonds are paramount in maintaining protein stability and proper folding. The presence of free thiol groups on cysteine residues, resulting from incomplete disulfide bond formation, can lead to improper protein folding and subsequent aggregation.
 Figure 1. The aggregation of ADC.
Figure 1. The aggregation of ADC.Implications and Control of Antibody Aggregation
Antibody aggregates poses significant risks to the efficacy and safety of therapeutic antibodies. Understanding the mechanisms of aggregate formation, such as structural disruption and disulfide bond formation, is essential for developing strategies to mitigate these aggregates. In-depth knowledge of these processes can inform regulatory standards and optimization of therapeutic formulations, thus ensuring product integrity and patient safety.
Regulatory Mechanisms for Antibody Aggregation
Antibody aggregation can significantly impact the quality and efficacy of therapeutic antibodies. During cell culture, various strategies can be employed to regulate antibody aggregate levels by manipulating the physicochemical environment. Key parameters include temperature, pH, and osmotic pressure, among others.
Environmental Factors in Cell Culture
Temperature Control: Studies, including those by Cromwell et al., have demonstrated that reducing the culture temperature can decrease the levels of antibody aggregates. Lower temperatures slow down the protein aggregation process by stabilizing the native structure of antibodies.
pH and Osmotic Pressure: The pH and osmotic pressure of the culture medium also play crucial roles. Elevated pH and osmotic pressure can reduce aggregate formation. For instance, increasing the concentration of sodium chloride in the culture medium enhances osmotic pressure, subsequently lowering aggregate levels.
Redox Modulating Agents
The addition of redox-active compounds, such as glutathione, cysteine, cystine, and copper sulfate, has been shown to minimize antibody aggregation during CHO cell culture. These agents can modulate the redox state, thereby preventing oxidative stress, which can lead to protein misfolding and aggregation.
Modulation of Protein Charge Distribution
Amino Acid Mutations: Altering the charge distribution on the antibody surface can reduce aggregation. This can be achieved by mutating exposed amino acid residues or adding charged peptides to the antibody termini. Specifically, introducing negatively charged amino acids increases electrostatic repulsion between proteins, thereby inhibiting aggregation.
Methionine Oxidation: Oxidation of methionine residues can influence the propensity for protein aggregation. To prevent methionine oxidation, freezing is often employed during antibody storage and transportation, effectively reducing aggregate formation.
Process Optimization and Genetic Engineering
Culture Duration: Shortening the culture duration can also lower aggregate content. When the desired yield is reached, minimizing culture time can effectively reduce aggregate formation.
pH and Ionic Strength Adjustment: The pH and ionic strength of the solution can affect the net charge on antibodies, enhancing electrostatic repulsion and thus preventing aggregation.
Genetic Modifications: Genetic approaches, such as mutating exposed amino acid residues or adding charged peptides to the antibody termini, can increase the surface charge of monoclonal antibodies. This enhances electrostatic repulsion, counteracting the strong hydrophobic interactions at "hot spots" that promote aggregation.
The formation of antibody aggregates can be mitigated through a combination of environmental controls, redox modulation, charge distribution alteration, and process optimization. By carefully managing these factors, the stability and efficacy of therapeutic antibodies can be preserved, ensuring their safe and effective use in clinical applications.
Techniques for Mitigating Antibody Aggregate Formation in Downstream Processes
Optimization of downstream purification processes is imperative when high levels of antibody aggregates are not sufficiently addressed during cell culture. Various methodologies can be implemented to reduce aggregate content effectively.
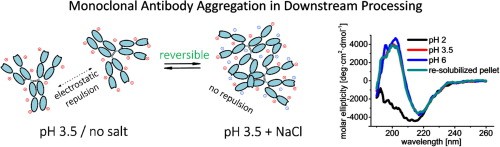 Figure 2. Monoclonal Antibody Aggregationin Downstream Processing
Figure 2. Monoclonal Antibody Aggregationin Downstream ProcessingBuffer Selection during Purification
The selection of buffer systems utilized in purification significantly influences aggregate levels. Acidic buffers, commonly employed during Protein A chromatography for the elution of antibodies, have been shown to induce aggregate formation. However, empirical evidence suggests that buffers containing arginine, in contrast to citrate, glycine, or histidine, markedly decrease the aggregate content in harvest solutions.
Size Exclusion Chromatography (SEC)
Size exclusion chromatography (SEC) is a highly effective technique for the separation of monomers from aggregates based on molecular size disparities. Although SEC can efficiently remove aggregates, a concomitant reduction in recovery yield is frequently observed.
Hydrophobic Interaction Chromatography (HIC) and Ultrafiltration
Both hydrophobic interaction chromatography (HIC) and ultrafiltration are valuable methodologies for aggregate removal. These techniques capitalize on differential hydrophobicity and molecular size, respectively, to separate monomeric antibodies from aggregated forms.
Anion Exchange (AEX) and Cation Exchange (CEX) Chromatography
Anion exchange (AEX) and cation exchange (CEX) chromatography are particularly effective in mitigating aggregate levels. These chromatographic techniques facilitate the dissociation of dimers and multimers into monomeric configurations. Under optimal conditions, these methods can reduce the aggregate content to below 0.5%.
By implementing these strategies, the aggregate content in biopharmaceutical products can be significantly reduced, thereby enhancing their safety and efficacy.
Effects of Aggregates on Therapeutic Efficacy and Immunogenicity
Antibody aggregation adversely affects the therapeutic efficacy and immunogenicity of monoclonal antibody-based drugs. The formation of aggregates during therapeutic administration can reduce drug efficacy and potentially induce immune responses in the host. Experimental investigations utilizing animal models have elucidated that both the structural characteristics and size of aggregates contribute to their immunogenic properties. The hypothesis that the immunogenicity of aggregates is contingent upon the repetitive antigenic epitopes on their surface has been supported by empirical data.
In conclusion, minimizing antibody aggregation is critical for optimizing both the efficacy and safety of monoclonal antibody therapeutics, as well as for controlling associated production costs. The implementation of rigorous analytical and quality control measures is imperative to mitigate the formation of aggregates during production and storage.
Process of Antibody Aggregation
Disruption of Native Protein Structure
The aggregation process commences with the disruption of the protein's native structure, leading to reversible self-association. This disruption can cause proteins to self-associate transiently, forming initial aggregates that may dissociate under certain conditions.
Irreversible Self-Association
Due to conformational changes, the reversible self-association can become irreversible. These structural alterations result in the formation of stable aggregates that are resistant to dissociation, contributing to the persistence of aggregated forms.
Growth and Formation of Soluble Aggregates
The addition of other monomers can increase the size of the aggregates. As aggregates interact with each other, they form soluble aggregate complexes, which further increase in size. These soluble aggregate complexes may subsequently separate from one another under certain conditions.
Formation of Insoluble Aggregates
Further conformational changes can lead to irreversible self-association, resulting in the production of insoluble aggregates. These insoluble forms are characterized by their inability to dissolve in the solution, thus complicating their removal and affecting product quality.
Prevention of Protein Aggregation During Purification
Batch Binding Experiments
In the case of high protein concentrations during chromatography, batch binding experiments can be conducted to assess whether the protein is in a saturated state. If randomly binding proteins do not aggregate, high-density binding may still induce aggregation. However, due to the impracticality of batch binding experiments, column chromatography remains a more reliable method for verification.
Use of Different Devices or Membranes in Ultrafiltration
In ultrafiltration processes, using different devices or membranes can be a method to assess aggregation tendencies. The presence of arginine in the protein solution during ultrafiltration has been shown to significantly inhibit aggregate formation. Similarly, arginine exhibits analogous effects in column chromatography.
Mechanisms Underlying Antibody Fragmentation
Antibody fragmentation can occur through both non-enzymatic and enzymatic pathways. Understanding these mechanisms is crucial for controlling the integrity of antibody products during cell culture and subsequent processing.
Non-Enzymatic Fragmentation
Non-enzymatic fragmentation is significantly influenced by the presence of metal ions, with copper ions (Cu²⁺) playing a pivotal role. The degree of antibody fragmentation increases with the concentration of Cu²⁺ in the environment. Experimental observations have demonstrated that antibodies incubated in phosphate-buffered saline (PBS) with various metal ions exhibit significantly higher fragmentation levels when Cu²⁺ is present, compared to other ions such as Mg²⁺, Mn²⁺, Zn²⁺, Fe²⁺, or Ni²⁺. Moreover, Fe²⁺ ions in the culture medium can participate in Fenton reactions, leading to the formation of hydroxyl radicals. This process elevates the levels of reactive oxygen species (ROS), which subsequently enhance antibody fragmentation. However, the addition of metal chelating agents, such as ethylenediaminetetraacetic acid (EDTA), can inhibit these processes by sequestering metal ions and thereby reducing fragmentation.
Additionally, other medium components, such as cysteine, zinc ions, and cobalt ions, have been found to reduce the extent of antibody fragmentation. These agents may exert protective effects by mitigating oxidative stress or stabilizing the antibody structure.
Enzymatic Fragmentation
The enzymatic fragmentation of antibodies involves two major redox enzyme systems: the glutathione system and the thioredoxin system. The relative contributions of these systems to antibody fragmentation can vary significantly depending on the specific conditions of the cell culture. In some instances, one system may predominate, while in others, both systems may act synergistically. Both the glutathione and thioredoxin systems require nicotinamide adenine dinucleotide phosphate (NADPH) to mediate the reduction of disulfide bonds within antibody molecules, thereby influencing their stability and integrity.
These redox enzyme systems are ubiquitously present in mammalian cells and are associated with a range of cellular functions, including antioxidative defense, metabolism, protein synthesis, amino acid transport, and cell cycle regulation. Disruption of genes encoding these enzymes can lead to profound phenotypic and functional changes in cells, potentially resulting in compromised cell viability.
Importance of Understanding Fragmentation Mechanisms
To effectively manage and minimize antibody fragmentation during cell culture, it is essential to elucidate the underlying mechanisms and factors contributing to this process. A particular focus should be placed on understanding the impact of nutritional components and other culture conditions on fragmentation. Such insights will enable the optimization of cell culture protocols and the development of strategies to preserve the structural integrity of antibody products, thereby ensuring their efficacy and safety.
Impact of Antibody Fragmentation on Therapeutic Efficacy and Safety
The fragmentation of antibodies can profoundly affect the safety and efficacy of antibody-based therapeutics. Specifically, the generation of antibody fragments, particularly those in reduced forms, introduces several potential adverse effects that may undermine both therapeutic outcomes and patient safety.
Diminished Biological Activity
Antibody fragmentation often results in diminished biological activity. The structural integrity of an antibody is essential for its capacity to bind to specific antigens with high affinity and specificity. The presence of fragments disrupts this structural integrity, which can impair the therapeutic antibody's ability to neutralize pathogens, inhibit target molecules, or induce desired immunological responses. Consequently, the therapeutic efficacy may be compromised.
Reduced Half-Life
The pharmacokinetic profile of therapeutic antibodies is negatively impacted by fragmentation. Intact antibodies generally possess extended half-lives in systemic circulation, facilitated by mechanisms such as neonatal Fc receptor (FcRn)-mediated recycling. Fragmented antibodies, however, lack the requisite structural elements for FcRn binding, leading to their accelerated clearance from the bloodstream. This reduction in half-life necessitates more frequent dosing, which increases the treatment burden on patients and may elevate healthcare costs.
Immunogenicity and Safety Concerns
Antibody fragments can also increase the risk of immunogenic responses. The immune system may recognize these fragments as foreign, leading to the generation of anti-drug antibodies (ADAs). The formation of ADAs can neutralize the therapeutic antibody's activity, diminish its efficacy, and, in certain cases, provoke hypersensitivity reactions or other adverse immune responses. This heightened immunogenicity poses a potential threat to patient safety, particularly for individuals with pre-existing immune sensitivities.
Clinical Implications
In light of these potential issues, it is crucial to minimize antibody fragmentation throughout the production, formulation, and storage phases of antibody therapeutics. Preserving the structural integrity of these biologics is vital for maintaining their therapeutic efficacy and safety. The development of robust analytical techniques for detecting and quantifying antibody fragments, alongside stringent quality control measures, is essential within the biopharmaceutical industry. Such measures are critical for preventing fragment formation and ensuring the delivery of therapeutically effective and safe products to patients.
Impact of Antibody Aggregation on Drug Efficacy
Elevated Costs Due to High Monoclonal Antibody Aggregation
The aggregation of monoclonal antibodies substantially influences the economic aspects of therapeutic antibody production by reducing purification yields and increasing overall production expenses. The presence of aggregates complicates the purification process and necessitates additional quality control measures, consequently escalating the manufacturing costs.
Methods for Detecting Antibody Fragments and Aggregates
Detection of Antibody Fragments
The detection of antibody fragments is commonly performed using Capillary Electrophoresis-Sodium Dodecyl Sulfate (CE-SDS) or Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE). CE-SDS provides a high-resolution separation of fragments based on size and charge, allowing for precise quantification. Similarly, SDS-PAGE is employed to resolve fragments according to their molecular weight, facilitating their identification and analysis.
Detection of Antibody Aggregates
The presence of antibody aggregates is typically assessed using SEC or SDS-PAGE. SEC separates aggregates based on their size, providing information on the distribution and concentration of aggregate species. SDS-PAGE can also be used to evaluate aggregate levels by resolving the aggregates alongside monomeric forms, enabling comparative analysis of aggregate content.
References
- Bickel F, Herold EM, Signes A, Romeijn S, Jiskoot W, Kiefer H. Reversible NaCl-induced aggregation of a monoclonal antibody at low pH: Characterization of aggregates and factors affecting aggregation. Eur J Pharm Biopharm. 2016 Oct
- Hansel, T., Kropshofer, H., Singer, T. et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9, 325–338 (2010)
- Li, W.; Prabakaran, P.; Chen, W.; Zhu, Z.; Feng, Y.; Dimitrov, D.S. Antibody Aggregation: Insights from Sequence and Structure. Antibodies 2016, 5, 19.











