In the development of antibody-based therapeutics, the establishment of cell lines is a critical step, influencing not only the production costs and efficiency but also the final product's quality and stability. For companies specializing in protein and antibody drug characterization, ensuring the efficiency and stability of cell lines is paramount to achieving success. As providers of protein and antibody drug characterization services, it is well understood that the quality of the cell line directly impacts the quality analysis, stability studies, and optimization of the production process for antibody drugs. Consequently, comprehensive support in cell line development and characterization is provided to antibody research and development enterprises, assisting clients in ensuring drug quality and consistency throughout the earliest stages of development. This article delves into the fundamental processes and key techniques in cell line development, offering essential support and guidance for all stages of pharmaceutical development.
The Importance of Cell Line Development
Host cells are at the core of the development and production of biopharmaceuticals. Among the 71 biotechnological drugs approved in the past four years, 62 contained recombinant proteins, of which 84% were derived from mammalian cells. Efficient and high-quality cell line development (CLD) is critical for the successful commercialization of biopharmaceutical products. Establishing a reliable CLD process is not a trivial task; the process is both costly and time-consuming, with the development of GMP-grade cell lines typically requiring 12 to 18 months. Not only must this process meet stringent regulatory standards, but each step of the development involves complex and meticulous analysis and quality control to ensure successful commercial production. Any lapse in this process can lead to significant financial losses, with delays often resulting in immeasurable costs. The development of high-yield, high-quality cell lines can reduce costs associated with upstream and downstream process optimization, while also accelerating the timelines for submitting IND (Investigational New Drug) and BLA (Biologics License Application) filings.
Basic Process of Cell Line Development
The construction of stable cell lines generally involves the following stages: vector construction, cell transfection, pool cell screening, monoclonal screening, cell bank establishment, and cell bank characterization. The entire development cycle typically spans 12 to 18 months. Improvements in any step of the standardized process can influence the overall CLD timeline.
The process is illustrated as follows:
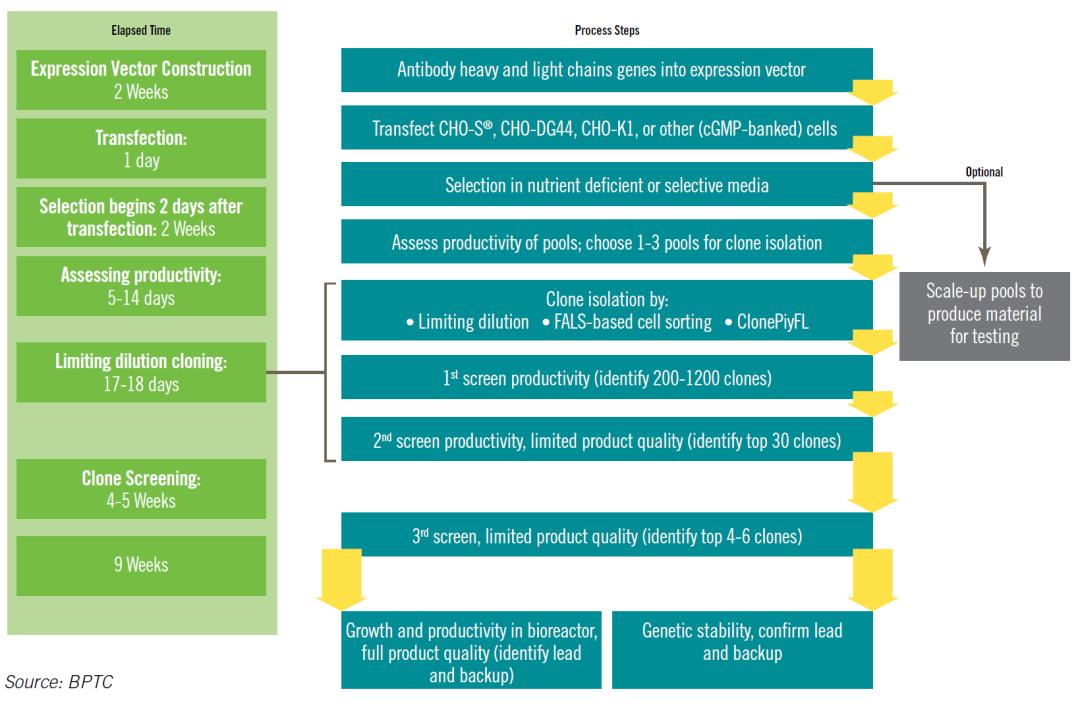
Evaluation Criteria for High-Quality Cell Lines
Cell line development (CLD) serves as the foundation for the Chemistry, Manufacturing, and Controls (CMC) of antibody drugs, directly influencing production costs, process complexity, and product quality. The quality of a cell line is a determining factor in the success of the commercial-scale production of biopharmaceuticals. High-quality cell lines typically meet the following criteria:
- High Yield: A high-yield cell line can reduce both fixed investment and production costs per batch.
- Superior Quality: Product quality is crucial for both efficacy and safety, which are key components of Investigational New Drug (IND) and New Drug Application (NDA) submissions.
- Stability: The yield and quality should remain consistent during passage and expansion.
- Scalability: The cell line must be adaptable to large-scale production systems.
- Compliance: Verification of monoclonal origin and the integrity of experimental records is essential. Additionally, the construction of the cell line should avoid exposure to or inclusion of animal-derived components or other substances that may pose a safety risk.
Selection of Host Cells
Mammalian cells are the predominant expression system used for clinical antibody products. To maintain the biological activity of therapeutic antibodies, correct folding and post-translational modifications are essential. As a result, mammalian cells, including Sp2/0 murine myeloma cells, NS0 murine myeloma cells, HEK293 human embryonic kidney cells, and Chinese hamster ovary (CHO) cells, are frequently employed in the production of therapeutic antibodies. Among these, CHO cells are the most widely used.
When compared to other expression systems, CHO cells offer several significant advantages:
- Suspension Culture Suitability: CHO cells are well-suited for suspension culture, meeting the demands of large-scale industrial production of recombinant proteins.
- Structural and Functional Similarity: Antibodies produced in CHO cells are structurally and functionally similar to native antibodies.
- Minimal Human Virus Contamination: CHO cells contain minimal human viral components, reducing potential safety concerns.
- Stable Integration of Foreign Genes: Foreign genes can be stably integrated into the CHO cell genome.
- Low Endogenous Protein Secretion: CHO cells are fibroblasts and secrete very little endogenous protein, which benefits the isolation and purification of target recombinant antibodies.
Criteria for Selecting Host Cells
The selection of host cells is based primarily on two considerations: safety and suitability. From a safety standpoint, the origin and cultivation history of the host cells must be well-documented, with traceable background information, such as the original institution where the cell line was isolated and established, the presence of any exogenous sequences, and the materials used in the cell's propagation. For newly developed or genetically modified cell lines, which lack comprehensive background data, there may be additional safety concerns. These risks require careful assessment and control, particularly when new risks related to tumorigenicity or other potential hazards have yet to be fully understood.
Monoclonality Verification
The verification of monoclonality is critical to ensuring the purity and uniformity of biopharmaceutical products. This verification is required by regulatory bodies, such as the FDA, to demonstrate the monoclonal origin of antibodies developed during the production process. Monoclonality verification must be rigorously documented and may be supported by various imaging technologies.
Several methods are employed in the industry to ensure monoclonality in cell line development:
- ClonePix for Monoclonal Selection: ClonePix is a high-throughput, automated system used to select monoclonal cell lines from semi-solid media. This method utilizes fluorescence-based imaging to identify high-expression clones, offering advantages in terms of reduced human intervention, higher throughput, and improved efficiency compared to traditional dilution methods. ClonePix integrates single-cell isolation with yield screening into a single step, significantly reducing screening time and increasing the number of candidates for further analysis.
- Single-Cell Printer: A newly introduced technology, the single-cell printer, improves efficiency and accuracy in monoclonal selection by reducing human dependence. The device separates cells into droplets, capturing high-resolution images to determine cell numbers within each droplet. This method significantly enhances the purity of monoclonal populations and increases the overall success rate of cloning by maintaining high cell viability.
- Fluorescence-Activated Cell Sorting (FACS): FACS is a method that can be optimized to select single-cell populations for monoclonality verification. It is highly efficient, with the potential for a success rate exceeding 99% when paired with imaging systems. FACS also allows for precise sorting based on cell morphology and fluorescence, although additional rounds of dilution may be required to further enrich monoclonality.
- Limiting Dilution Method: Limiting dilution is a simpler method that requires minimal equipment and can be easily combined with imaging systems. While it is widely used, its efficiency is relatively low, requiring large-scale screening using 96-well or 384-well plates. Limiting dilution depends heavily on manual operation, which can introduce variability depending on the operator's technique. To increase consistency, automated workstations can be employed for more reproducible cell dilution practices.
Each of these methods provides a means of enhancing monoclonality verification, with varying degrees of efficiency, cost, and complexity. The choice of method depends on specific project needs, available equipment, and desired outcomes.
Principles of Cell Line Selection
The principle underlying cell line selection involves the transfection of host cells with plasmids carrying selective markers, such as metabolic markers or antibiotic resistance markers. Upon transfection, the plasmids are introduced into the host cells, and selective media are employed to identify successful clones. Host cells that either fail to receive the plasmid or integrate it improperly will not be able to survive in the selective medium due to the absence of the selective marker, while positively transfected clones will proliferate under these conditions, thereby facilitating the identification of successful clones.
In the amplification and selection process, selective pressure is applied to the transfected cells, ensuring that only those expressing high levels of the foreign gene survive. This selective pressure increases the expression of the target protein, allowing for the isolation of high-expression, stable cell lines. For example, methotrexate (MTX), an inhibitor of dihydrofolate reductase (DHFR), is used to amplify genes in cells lacking DHFR activity, such as CHO-DG44 cells. The presence of DHFR in the transfected vector allows cells to increase the copy number of both the DHFR gene and the target protein gene upon MTX exposure. By gradually increasing the MTX concentration, the gene copy number can be amplified until no further increase is observed, indicating the achievement of a maximum stable gene copy number.
In vector construction, weaker promoters can be used to reduce DHFR gene expression, or the DHFR gene itself can be engineered to be unstable, further enhancing the selection and amplification effects of MTX. A similar metabolic inhibitor, MSX, specifically inhibits glutamine synthetase (GS) and can be used as an alternative to the DHFR system. However, the GS-based selection system is less frequently used for monoclonal antibody (mAb) production due to concerns over its stability, as GS expression tends to decrease over time, potentially compromising the stability required for long-term production.
The G418 selection system is another widely used method. G418, an aminoglycoside antibiotic, inhibits protein synthesis in mammalian cells by interfering with ribosomal function. Cells expressing the neo gene, which confers resistance to G418, can survive in media containing this antibiotic. When a plasmid containing the neo resistance gene is transfected into mammalian cells, successful integration of the neo gene into the host genome will confer resistance, allowing these cells to proliferate in the presence of G418. Cells that fail to integrate the neo gene will not survive, allowing for the selection of positive clones.
Establishment of Cell Banks
Cell banks are classified according to the stage of research and their intended use, including Research Cell Banks (RCB), Production Cell Banks (PCB), Master Cell Banks (MCB), Working Cell Banks (WCB), and End-of-Production Cell Banks (EOPCB). A common categorization reflects the cell bank's purpose and whether it is maintained under Good Manufacturing Practice (GMP) conditions. These categories include non-GMP cell banks (RCB, PCB), GMP non-production cell banks (e.g., cell banks used for biological assays), and GMP production cell banks.
For biopharmaceutical researchers, expertise in establishing cell banks is crucial, whether for GMP production or for maintaining cell lines during research phases. The establishment of cell banks ensures the stability and consistency of recombinant monoclonal antibody production. Cell banks are typically organized into three levels: the Original Cell Bank (PCB), the Master Cell Bank (MCB), and the Working Cell Bank (WCB).
The Original Cell Bank (PCB) consists of a homogeneous population of recombinant cells, cloned and cultured to ensure uniformity. After validation, cells are aliquoted into ampoules and stored at cryogenic temperatures (liquid nitrogen or below -130°C) for future use in generating the MCB. The Master Cell Bank (MCB) is established by propagating cells from the PCB under controlled conditions. The cells are then mixed into a homogeneous batch, aliquoted, and stored under the same cryogenic conditions. The MCB is fully validated and serves as the source for generating the WCB.
The Working Cell Bank (WCB) is derived from the MCB through successive passages until the desired passage level is reached. These cells are then pooled into a homogeneous suspension, aliquoted, and stored under cryogenic conditions for use in production. The WCB serves as the main source for producing recombinant products. It is critical that the WCB be restricted to a single passage level, as the passage number must be controlled to ensure that, upon thawing, the cell quantity is sufficient for manufacturing and that the cell line remains within the approved passage limit for production.
Storage and Quality Control of Cell Banks
The integrity and consistency of cell banks are maintained through rigorous quality control processes, which include testing for sterility, genetic stability, and identity confirmation. For example, the genetic stability of the cell line is assessed through periodic analysis to verify that the transfected genes remain stable over multiple generations. Additionally, testing for contaminants, such as mycoplasma or endotoxins, is a critical component of cell bank maintenance.
In summary, the establishment and maintenance of cell banks are fundamental to ensuring the reproducibility, stability, and safety of recombinant protein production, especially in the context of large-scale biopharmaceutical manufacturing. Careful management of these cell banks, along with the application of appropriate selection systems, supports the long-term viability of the production process and the consistency of therapeutic products.
Study of Cell Line Stability
The primary aim of cell line stability studies is to determine the cell line's passage limit, ensuring that the quality and yield of its expressed products meet the anticipated requirements within the defined passage range. The requirements for cell line stability studies differ between the Investigational New Drug (IND) and Biologics License Application (BLA) stages.
At the IND Stage: Cell line stability studies are typically conducted using the Original Cell Bank (PCB), and these studies may be performed at small scale, either in shake flasks or bioreactors. The experimental procedure involves subculturing PCB seed cells under specific conditions, freezing cells at various passage levels (e.g., 5th, 10th, 15th, and 20th passages), and subsequently thawing and culturing the cells with the current production process. The focus during this stage is on assessing the stability of cell production, metabolism, and yield across the different passages. If appropriate analytical methods have been developed, quality attributes may also be evaluated simultaneously.
At the BLA Stage: Stricter requirements are imposed, and stability studies must be conducted using the Master Cell Bank (MCB) and Working Cell Bank (WCB) in larger-scale production or validated scaled-down models. The experimental process follows the same methodology as the PCB studies, involving subculturing the MCB and WCB seed cells under specific conditions, freezing cells at various passages, and then thawing and culturing them using commercial-scale production processes. At this stage, the focus is expanded to include the assessment of cell production, metabolism, yield, protein quality, and genetic stability across the various passages.
In situations where cell line stability is uncertain, it is recommended to conduct two sets of experiments: one with selective pressure and one without, to evaluate the stability of the cell line under different conditions.
Cell Line Qualification
Qualified production cells form the foundation for producing acceptable recombinant antibodies. To ensure the safety and efficacy of the final product, a comprehensive qualification of production cells is essential. This includes the identification of the cells and testing for microbial contamination. Recombinant monoclonal antibody (mAb) production cells, obtained through DNA recombinant technology and containing specific gene sequences, necessitate additional quality control measures. These include an examination of cell line stability, antibody gene or protein identification tests, and the detection of foreign viral contaminants in the cell culture.
Cell Line Screening Criteria and Analytical Methods
Clone screening typically focuses on titer levels and the antibody expression rate per cell per unit time (Qp). The clones selected should have Qp values that fall within the mid-range of all clones evaluated. Industrially, the Qp values for chosen clones typically range from 20 to 100 pg/cell/day. Additionally, the growth characteristics of the clone must be considered, with CHO cells in industrial settings typically doubling every 15 to 24 hours. During batch or fed-batch cultures, the growth phase should be extended as much as possible.
Once a clone reaches the shake flask stage, a Research Cell Bank (RCB) should be established for stability testing. Stability assessments generally involve subculturing the clone for up to 60 passages and evaluating several factors:
- Growth Stability: The consistency of the clone's doubling time.
- Expression Stability: The consistency of titer or Qp across passages.
- Genetic Stability: The maintenance of the transfected genes without significant loss or mutation.
- Product Quality Stability: Whether there is any significant difference in the quality of the antibodies expressed in different passages.
In addition to these evaluations, further analyses are conducted to ensure the genetic and functional integrity of the antibody. These include:
- cDNA Sequence Verification: To confirm that the antibody's amino acid sequence has not undergone mutations.
- Peptide Mapping: To verify the sequence and structure of the expressed antibody.
- CE-SDS and SDS-PAGE: To assess the purity of the mAb.
- HPLC-SEC: To eliminate clones that tend to form aggregates.
- Glycosylation Profiling: To identify clones with suboptimal glycosylation patterns.
- Isoelectric Focusing (IEF) or Capillary Isoelectric Focusing (iCIEF): To detect any acid or base isoforms of the antibody.
- Ion Exchange Chromatography HPLC: To ensure that high-acid or high-base variants are avoided.
Following shake flask evaluations, selected clones are scaled up in bioreactors to assess cell growth, monoclonal antibody expression, and product quality at production scale. A high-performing cell line should exhibit both stability and monoclonality, with protein quality taking precedence over yield.
You may interested in
The Role of Protein Characterization in High-Efficiency Cell Line Development
In the development of high-efficiency cell lines, it is crucial to ensure not only the stability and expression capacity of the cells but also the final quality of the protein drug. The quality of protein-based drugs directly impacts clinical efficacy and patient safety, making high-quality protein characterization services indispensable in the pharmaceutical development process. Through detailed protein analysis, critical attributes such as structural integrity, glycosylation patterns, and other key quality characteristics can be assessed. This enables clients to ensure the consistency and stability of each product batch throughout the development process, ultimately facilitating the progression into clinical phases and market approval.
By implementing thorough protein characterization protocols, the integrity and reproducibility of therapeutic protein production are maintained, ensuring that the final product meets the highest standards of quality and safety for clinical use.








