Antibody-based therapeutics are emerging as highly promising treatments in modern medicine, demonstrating substantial efficacy across various disease categories. Their precise targeting mechanisms allow for specific engagement with disease-associated targets, thereby facilitating effective therapeutic outcomes. Particularly noteworthy among these targets are molecules such as tumor necrosis factor-alpha (TNF-α), vascular endothelial growth factor (VEGF), CD20, epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor 2 (HER2), which play critical roles in inflammation, tumorigenesis, and immune modulation. This review comprehensively explores the biological mechanisms and market applications associated with these key therapeutic targets.
TNF-α
Mechanism Elucidation: TNF-α is a pivotal pro-inflammatory cytokine integral to normal inflammatory and immune responses. It is predominantly produced by activated monocytes and macrophages, existing in both a transmembrane form (tmTNF) and a soluble form (sTNF). tmTNF, distributed on the surface of TNF-α-secreting cells as a membrane protein, is converted to sTNF through activation by the TACE enzyme. TNF-α receptors, categorized as TNFRI and TNFRII, are ubiquitously expressed on various cell surfaces. The binding of TNF-α to its receptors triggers biological effects such as apoptosis, inflammation, and tumor progression. Dysregulation of TNF-α is implicated in numerous diseases, including but not limited to cardiovascular diseases, cachexia, septic shock-induced multi-organ failure, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, graft-versus-host disease, and bone marrow hematopoietic disorders. TNF-α inhibitors function by targeting and neutralizing both sTNF and tmTNF, thereby preventing their interaction with TNF receptors and inhibiting TNF-mediated biological activity. Additionally, the reverse signaling effect induced by TNF-α inhibitors binding to tmTNF can directly affect cells secreting excessive TNF-α, thus mitigating autoimmune attacks at their source.
Market Overview: Globally, several macromolecular drugs targeting TNF-α have been approved and are commercially available. These include infliximab, adalimumab, certolizumab, golimumab, and etanercept, all of which act on TNF-α.
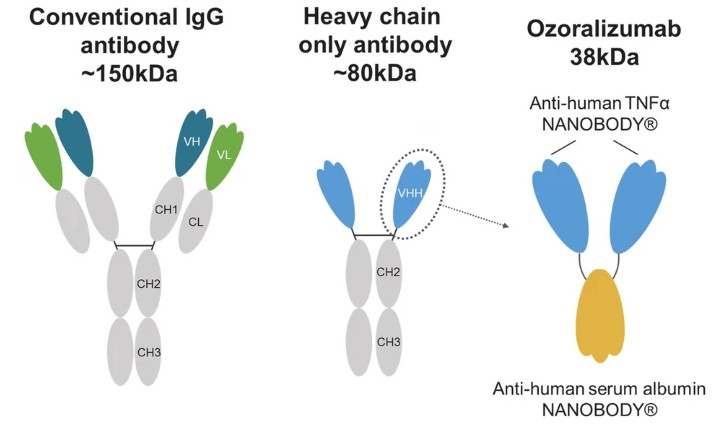 Figure 1. Schematic representation of a conventional IgG antibody, heavy-chain-only antibody, and ozoralizumab.
Figure 1. Schematic representation of a conventional IgG antibody, heavy-chain-only antibody, and ozoralizumab.VEGF
Mechanism: VEGF, a pivotal intracellular signaling protein, plays a critical role in stimulating angiogenesis, demonstrating significant pro-angiogenic and regenerative functions. Its mechanism involves specific binding to vascular endothelial growth factor receptors (VEGFRs), also known as tyrosine kinase receptors, on the cell membrane. Subsequently, through a series of intricate signaling pathways, VEGF triggers biological responses that promote vascular formation. As early as 1971, Folkman proposed the theory of tumor angiogenesis, highlighting the tumor's reliance on vascular growth. Targeting this mechanism, VEGF (VEGFR) antibody therapies selectively bind to VEGF (VEGFR), thereby blocking downstream signaling pathways and inhibiting angiogenesis.
Market: Bevacizumab, the first humanized monoclonal antibody targeting the VEGF receptor, has successfully inhibited tumor vascular proliferation due to its high affinity and specific binding to VEGF. In 2004, Genentech's bevacizumab (trade name Avastin) received FDA approval for treating metastatic colorectal cancer. Moreover, targeted therapies against abnormal angiogenesis involved in solid tumors, age-related macular degeneration (AMD), and other pathogenic mechanisms have shown significant therapeutic efficacy targeting the VEGF vascular signaling pathway. Currently, FDA has approved several monoclonal antibodies or fusion proteins targeting VEGF/VEGFR, including bevacizumab, ranibizumab, aflibercept, and ramucirumab. Ranibizumab, a monoclonal antibody fragment targeting VEGF, was developed by Genentech and first FDA-approved in 2006 under the trade name Lucentis, primarily used in the treatment of retinal macular degeneration. Lucentis, as a Fab fragment of Genentech's VEGF antibody Avastin, shares a mechanism of action consistent with Avastin.
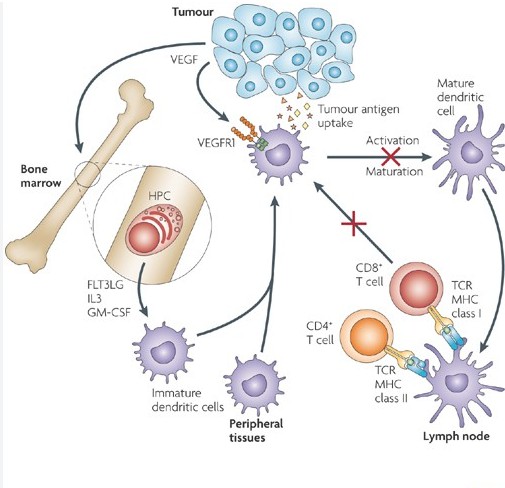 Figure 2. Mechanisms of anti-tumour activity of VEGF-targeted therapy
Figure 2. Mechanisms of anti-tumour activity of VEGF-targeted therapyCD20
Mechanism: CD20, a transmembrane phosphoprotein, is specifically located on the surface of B lymphocytes. The development of B lymphocytes originates from multipotent stem cells in the bone marrow, progressing through several stages including pro-B cells, pre-B cells, immature B cells, and ultimately mature B cells. Upon release into lymphoid tissues, mature B cells can further differentiate into plasma cells. Notably, CD20, serving as a surface antigen of B cells, is expressed exclusively from the pre-B cell to mature B cell stages, but not on hematopoietic stem cells, progenitor B cells, or mature plasma cells. In humans, the gene encoding the CD20 surface antigen is MS4A1. Besides normal B cells, CD20 is also expressed on tumor cells derived from B cells, such as lymphomas and leukemias, as well as B cells involved in immune and inflammatory diseases. Consequently, CD20 has emerged as a significant therapeutic target for lymphomas, leukemias, and certain autoimmune diseases.
Mechanism of Action: Anti-CD20 monoclonal antibodies are monoclonal antibodies targeting CD20. While the complete functions of CD20 are not fully elucidated, existing evidence suggests that anti-CD20 monoclonal antibodies exert cytotoxic effects against B cell-derived tumors through three mechanisms: antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and direct effects induced by antibody binding to CD20 molecules, such as growth inhibition, cell cycle alteration, and apoptosis.
Market: The market for anti-CD20 monoclonal antibodies has rapidly expanded, with several drugs targeting CD20 currently available. These include rituximab (brand name: Rituxan), ofatumumab (brand name: Arzerra), obinutuzumab (brand name: Gazyva), trastuzumab (brand name: Herceptin), and others. These drugs are widely used in the treatment of lymphomas, leukemias, and certain autoimmune diseases.
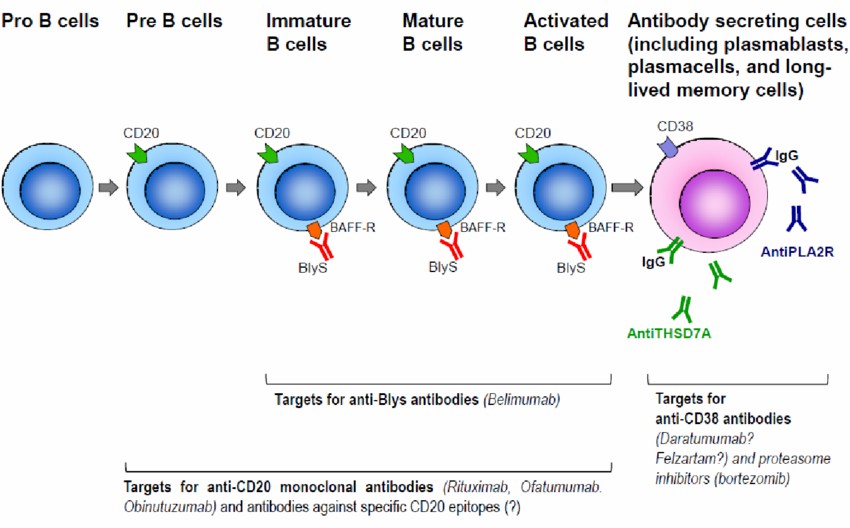 Figure 3. Targets for anti CD20 monoclonal antibodies in B cell lineages.
Figure 3. Targets for anti CD20 monoclonal antibodies in B cell lineages.EGFR
Mechanism of EGFR: EGFR, belonging to the receptor tyrosine kinase I subfamily, encompasses other members such as HER2/neu, HER3, and HER4. These receptors share a distinct structural framework consisting of an extracellular ligand-binding domain, a single-pass transmembrane region, and an intracellular tyrosine kinase domain. The phenomenon of EGFR overexpression has been documented across a spectrum of human tumors. The EGFR signaling pathway assumes pivotal roles in tumor cell proliferation, repair of cellular damage, invasion, and angiogenesis. Consequently, the development of EGFR-targeted therapies remains a central focus in oncology research.
Market Overview: Currently, targeted therapies for EGFR primarily fall into two categories. One category consists of large-molecule monoclonal antibodies, including cetuximab, panitumumab, and necitumumab. The other category comprises small molecule inhibitors such as gefitinib, erlotinib, afatinib, dacomitinib, osimertinib, and others. These drugs play significant roles in cancer treatment.
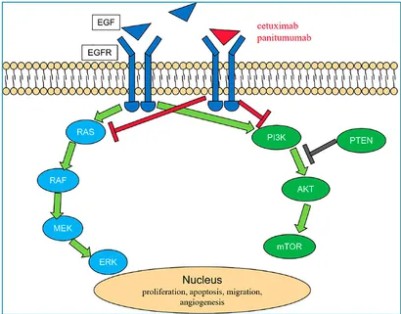 Figure 4. EGFR pathway.
Figure 4. EGFR pathway.HER2
Mechanism: HER2 monoclonal antibodies are key treatments for HER2-positive breast and gastric cancers, targeting the HER2 protein. HER2 protein is widely expressed in breast cancer and gastric/esophageal cancers as a transmembrane protein with tyrosine kinase activity, belonging to the EGFR family. The structure of this protein includes an extracellular ligand-binding domain, a single-pass transmembrane region, and an intracellular protein tyrosine kinase domain. Despite no directly bound ligands discovered, HER2 protein primarily forms heterodimers with EGFR (HER1/erbB1), HER3/erbB3, HER4/erbB4 family members, subsequently binding to respective ligands. HER2 protein plays a crucial role in signal transduction pathways, including Ras/Raf/MAPK, PI3K/Akt, STAT, and PLC. Approximately 25% to 30% of breast cancers and 10% to 30% of gastric/esophageal cancers exhibit HER2 gene amplification or overexpression. Moreover, HER2 overexpression is also observed in ovarian, endometrial, bladder, colorectal, and head and neck cancers. Due to its overexpression in tumors and extracellular domain characteristics, HER2 has become a crucial target for antibody drug development.
Market: As of now, FDA has approved five HER2 monoclonal antibody drugs, including trastuzumab, pertuzumab, and T-DM1 under Roche's portfolio. Sales of Roche's HER2 monoclonal antibodies continue to rise, with trastuzumab's annual sales exceeding 7 billion Swiss francs, driven by combination therapies with pertuzumab. Additionally, Ogivri, the first biosimilar to trastuzumab approved by the FDA, and Margetuximab, an Fc-modified HER2 monoclonal antibody approved by MacroGenics in January 2018, have injected new vitality into this market.
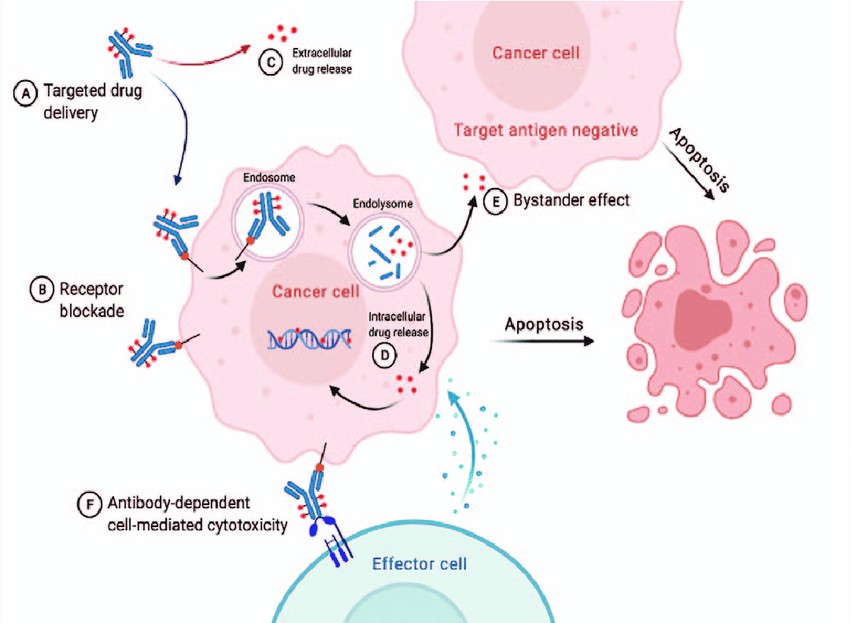 Figure 5. The main mechanism of ADCs targeting HER2.
Figure 5. The main mechanism of ADCs targeting HER2.The Importance of Antibody Drug Characterization
The characterization of antibody drugs plays a crucial role in their development process. Thorough analysis and characterization of antibody structure, binding properties, potency, and stability ensure both efficacy and safety of the drug. Below are key aspects of antibody drug characterization services for various targets:
For TNF-α targeted antibody drugs, characterization services are essential in evaluating affinity to TNF-α, ability to neutralize TNF-α activity, and stability under different conditions. These analyses optimize antibody drug design and ensure efficacy and safety in clinical applications.
In the case of VEGF targeted antibody drugs, characterization involves assessing binding properties to VEGF, efficacy in inhibiting angiogenesis, and pharmacokinetic properties in vivo. These results guide drug development to enhance therapeutic outcomes in tumor treatment.
In the development of CD20 targeted antibody drugs, characterization services evaluate specific binding to B cells, cytotoxicity, stability, and immunogenicity both in vitro and in vivo. These analyses provide critical data support for the design and optimization of anti-CD20 monoclonal antibodies.
For EGFR targeted antibody drugs, characterization services include affinity and specificity analysis to EGFR, efficacy testing in inhibiting tumor cell proliferation, and stability evaluation under varying conditions. Such analyses ensure the efficacy and safety of anti-EGFR drugs in clinical applications.
Regarding HER2 targeted antibody drugs, characterization services involve evaluating binding properties to HER2, capacity to inhibit HER2 signaling pathways, and pharmacokinetics and stability in vivo. These analyses provide robust data support for the development and clinical application of HER2 antibody drugs.
You may interested in
Summary
The development of antibody drugs has brought new hope for the treatment of numerous diseases, particularly in the fields of oncology and immune-related disorders. Antibody drugs targeting specific proteins have demonstrated significant clinical efficacy and market potential. The successful applications targeting TNF-α, VEGF, CD20, EGFR, HER2, and other receptors signify that antibody drug research has entered a mature stage. However, with ongoing advancements in scientific technology, future discoveries and utilization of new targets are anticipated.
Comprehensive antibody drug characterization services allow researchers to gain profound insights into the properties of these drugs, optimize their design, enhance the success rates of clinical trials, and ensure competitiveness in the market. Such services not only provide scientific foundations for drug development but also expedite the regulatory approval process, thereby offering patients more effective treatment options.
References
- Oyama, S., Ebina, K., Etani, Y. et al. A novel anti-TNF-α drug ozoralizumab rapidly distributes to inflamed joint tissues in a mouse model of collagen induced arthritis. Sci Rep 12, 18102 (2022).
- Ellis, L., Hicklin, D. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8, 579–591 (2008).
- RUGGENENTI, Piero; REMUZZI, Giuseppe. Disease-Specific Treatment for Primary Membranous Nephropathy: The Role of Monoclonal Antibodies. Medical Research Archives, 2023.
- Meiqin Yuan, Zeng Wang, Wangxia, Hongming Pan. The Role of Anti-EGFR Monoclonal Antibody in mCRC Maintenance Therapy. Molecular Diagnostics and Therapeutics, 30 March 2022
- Li, Lixi; Zhang, Di; Liu, Binliang; Lv, Dan; Zhai, Jingtong; Guan, Xiuwen; Yi, Zongbi; Antibody-drug conjugates in HER2-positive breast cancer. Chinese Medical Journal 2022.












