The developmental history of bispecific antibodies (BsAbs) can be delineated into five distinct phases:
Phase I: In 1960, Nisonoff and colleagues at the Memorial Sloan Kettering Cancer Center in New York, in a seminal publication in Science, first proposed the concept of bispecific antibodies.
Phase II: By the 1980s, Morrison et al. pioneered the production of the first tetravalent BsAb capable of binding both hapten and dinitrophenyl groups. This achievement was made possible by linking different specificities of single-chain antibodies via flexible peptide segments and subsequent fusion expression.
Phase III: During the 1990s, Medarex, Inc. successfully developed bispecific antibodies and initiated Phase III clinical trials by 2001. However, setbacks in clinical trials and production challenges temporarily stalled research in this field.
Phase IV: The first BsAb drug, catumaxomab (Removab), received European Union approval in 2009 for the treatment of malignant ascites but was withdrawn from the market in 2017.
Phase V: With advancements in antibody engineering, particularly through phage display technology, the development of bispecific antibodies has seen renewed opportunities. Blinatumomab (Blincyto), approved by the FDA and EMA in 2014, marked a significant milestone. Subsequently, emicizumab (Hemlibra) gained FDA approval in 2017 for routine prophylaxis in hemophilia A, and more recently, amivantamab received FDA approval for the treatment of non-small cell lung cancer.
Definition and Advantages of Bispecific Antibodies
What are Bispecific Antibodies
BsAbs, a type of engineered antibodies, specifically refer to artificial antibodies with dual antigen-binding sites. These antibodies serve as bridges between target cells and effector molecules, thereby inducing targeted immune responses. Currently, BsAbs have emerged as a focal point in the field of antibody engineering, particularly demonstrating extensive potential in the realm of tumor immunotherapy.
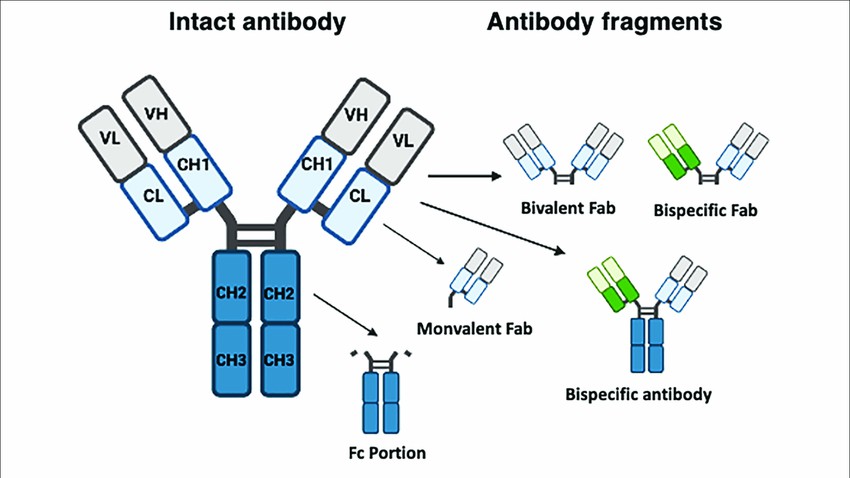 Figure 1. Typical structure of monoclonal and bispecific antibodies.
Figure 1. Typical structure of monoclonal and bispecific antibodies.Advantages of Bispecific Antibodies
The advantages of BsAbs are delineated as follows:
Enhanced efficacy: Compared to conventional monoclonal antibodies, BsAbs can simultaneously bind two distinct epitopes or target proteins, thereby exerting unique biological effects that conventional monoclonal antibodies cannot match.
Reduced toxicity: BsAbs exhibit significantly reduced off-target toxicity due to their enhanced specificity and targeting capabilities, thereby enhancing treatment safety.
Cost-effectiveness: BsAb therapies are more cost-effective compared to combination therapies involving monoclonal antibodies.
Structural properties: BsAbs containing the Fc region demonstrate excellent solubility, stability, and prolonged half-life, while also enhancing antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), thereby improving tumor-killing efficacy. Fc-less BsAbs, due to their smaller size and substantially reduced dosages (approximately less than 1/100th of conventional antibodies), offer unique advantages.
Resistance prevention: By simultaneously targeting dual epitopes and blocking dual signaling pathways, BsAbs effectively prevent the development of resistance mechanisms. Notably, receptor tyrosine kinases (RTKs), such as those from the Her family, play crucial roles in cellular proliferation, highlighting the significant impact of BsAbs on such targets.
In summary, bispecific antibodies, with their cost-effectiveness, enhanced efficacy, and promising market prospects, have become a prominent technology in current biomedical research.
Classification of Bispecific Antibodies
Fc-Containing Bispecific Antibodies:
Structurally and functionally, Fc-containing bispecific antibodies (Fc-BsAbs) exhibit notable distinctions from Fc-less bispecific antibodies. The primary characteristic of Fc-BsAbs is the retention of the Fc region, aligning them closely with native antibody architectures. This design enables them to facilitate biological activities like ADCC and CDC. However, despite their functional resemblance to natural antibodies, variations in size and structure may impair desirable attributes such as stability and solubility, thereby influencing their physicochemical and pharmacokinetic properties.
Fc-Less Bispecific Antibodies:
In contrast, Fc-less bispecific antibodies demonstrate distinctive structural and functional characteristics attributable to the absence of an Fc region. This design feature reduces their molecular mass, facilitating expression in prokaryotic cells and improving penetration into tissues and tumor cells for enhanced target site accessibility. However, this structural modification also precludes the facilitation of biological functions reliant on the Fc region and typically results in a shorter half-life. For instance, the approved antibody Blinatumomab exhibits a plasma half-life of only 2.11 hours, necessitating continuous 28-day infusion via a pump. Fragment-based bispecific antibodies integrate multiple antibody fragments within a single molecule, mitigating chain-related complexities and offering benefits such as increased yield and reduced production costs. Nonetheless, they are characterized by relatively short half-lives and susceptibility to stability and aggregation challenges.
Preparation Technologies of Bispecific Antibodies
Fc-Containing Bispecific Antibodies:
Triomabs: Triomabs engage tumor cells and T cells separately via their Fv regions, while recruiting FcR-expressing effector cells such as NK cells, monocytes, macrophages, neutrophils, and dendritic cells through their Fc regions to form complexes. This complex stimulates T cells to secrete cytokines, thereby eliminating tumor cells. Triomabs are known in the industry as trispecific antibodies and were jointly developed by Fresenius and Trion Pharma in Germany.
Knobs-into-holes Technology: Developed by Genentech, this technique enhances assembly efficiency by mutating antibody structures. Specifically, it involves mutating threonine (T) at position 366 in the heavy chain CH3 domain of one antibody to tyrosine (Y), forming a "knobs" structure, and mutating tyrosine (Y) at position 407 in the CH3 domain of another antibody to threonine (T), forming a "holes" structure. This spatial hindrance effect ensures correct assembly between different antibody heavy chains. Implementation of this technology has increased assembly rates from 57% in wild-type antibodies to 92%, meeting the demands of large-scale production.
Crossmab Technology: Represented by Roche's RG7221 and RG7716, Crossmab technology produces bispecific antibodies targeting Ang-2/VEGF. Based on the "knobs-holes" technology, it utilizes chain exchange to swap the CL and CH1 domains of the Ang-2 antibody's Fab structure, while maintaining the Fab structure of the VEGF antibody. This modification reduces mispairing between the light chain of the Ang-2 antibody and the heavy chain of the VEGF antibody, while the "knobs-holes" structure promotes heterodimerization of the two heavy chains.
Fc-Less Bispecific Antibody Preparation Technologies:
BiTE Bispecific Antibodies: Developed by Micromet (Germany), BiTE antibodies exemplify Fc-less bispecific antibodies. These antibodies are constructed by linking a single-chain variable fragment (scFv) targeting CD3 with another scFv targeting various tumor cell surface antigens via a peptide linker. BiTE antibodies can simultaneously bind CD3-positive T cells and tumor cells. By engaging CD3 on T cell surfaces, they redirect T cells towards tumor cells, thereby activating T cells to execute tumor-killing functions. Researchers using BiTE technology successfully addressed challenges such as poor scFv stability, low expression levels, and solubility issues, thus advancing the commercialization of BiTE products.
DART Bispecific Antibodies: DART technology, jointly developed by MacroGenics and Servier, represents a milestone in the construction of a new generation of bispecific antibodies. DART antibodies form heterodimeric antibodies by combining two peptide chains, each linking the VH sequence of one antibody's variable region with the VL sequence of another antibody's variable region. Additionally, this technology introduces cysteines at the C-terminus of the two peptide chains, forming interchain disulfide bonds that significantly enhance product stability.
bi-Nanobody Bispecific Antibodies: Developed by Ablynx using Nanobody technology based on single-domain antibodies from camels and llamas (lacking light chains and CH1 region), bi-Nanobody employs a simplified structure retaining only the VH domain, thus creating a unique patented platform. In practical applications, this technology links VH regions from two or more antibody molecules to achieve multispecific binding. bi-Nanobody's key advantages include small size, high stability, ease of humanization, ease of conjugation, and support for multiple administration routes. Moreover, by incorporating functional domains that bind to human serum albumin during molecular design, this technology effectively extends drug half-life to 2-3 weeks and facilitates precise drug delivery to target sites via albumin.
Mechanisms of Action of Bispecific Antibodies
Recruitment of T cells or natural killer cells (NK cells) and redirection towards tumor cells to enhance their cytotoxic capability against tumor cells. Simultaneously, BsAbs block distinct or overlapping signaling pathways involved in disease progression, thereby influencing tumor cell growth, proliferation, and survival. Additionally, by targeting different antigens or epitopes on cell surfaces, they enhance specific binding to tumor cells, facilitating direct tumor cell destruction.
Cell bridging mechanism: Based on redirecting T cells for specific killing of tumor cells. T cells establish physical connections with tumor cells through BsAbs composed of T cell-binding domains and tumor-binding domains. For instance, approved antibodies like Catumaxomab (approved in 2009) and Blinatumomab (approved in 2014) employ this mechanism. NK cell bridging: NK cells, crucial effector cell subsets, are also pivotal in BsAb design. BsAbs that pair antibodies targeting CD16a with those targeting tumor-associated antigens redirect NK cells to achieve anti-tumor effects.
Bridging receptor action: The development of diseases like cancer often involves multiple signaling pathways. Blocking a single pathway may inadequately suppress disease progression and could activate compensatory pathways. BsAbs can specifically block multiple signaling pathways, thereby achieving superior disease suppression effects.
Mediation of protein complex formation: Emicizumab, a humanized bispecific IgG4 antibody approved in 2017, targets both coagulation factors IXa and X. By simultaneously bridging these factors, it mimics the physiological function of factor VIII, thereby promoting thrombin generation.
Risks in Bispecific Drug Development
Cytokine Storm: Cytokine storm represents a significant adverse effect under the cell bridging mechanism of BsAbs, stemming from the high affinity of CD3 and Fc-mediated immune responses. The high affinity of CD3 can lead to premature activation of T cells by BsAbs before binding to tumor cells, triggering rapid cytokine release and resulting in a cytokine storm. Moreover, the high CD3 affinity accelerates drug metabolism, reducing BsAbs distribution within tumors and weakening their efficacy. Additionally, the Fc structure of BsAbs can bind to Fc receptors on other effector cells, also inducing cytokine storms.
Immunogenicity: The non-natural molecular structure of bispecific antibodies significantly increases the risk of immunogenicity, thereby compromising drug safety and efficacy. This process may generate anti-drug antibodies (ADAs) and neutralizing antibodies, potentially leading to severe drug-related toxic reactions or allergic responses through the formation of drug/ADA immune complexes. The immunogenicity of bispecific antibodies is influenced by various factors, including product-related impurities, antibody origin (human, chimeric), dosing regimen, and target molecules.
Poor Pharmacokinetics: The complex structure of bispecific antibodies limits their applicability in many monoclonal antibody drug development platforms, thereby increasing the complexity of drug development. During development, challenges such as protein fragmentation, aggregation, and multiple glycoforms are frequently encountered, posing significant challenges for analytical method development.
Strategies in Bispecific Drug Design
01 Overview of Four Main Functions:
- Redirecting specific immune effector cells to selectively destroy cancer cells;
- Targeting multiple cell surface antigens to enhance specificity;
- Achieving drug delivery into tumor interiors;
- Blocking two biological pathways to enhance treatment efficacy and durability.
02 Characteristics of IgG-like bsAbs:
IgG-like bsAbs, characterized by their Fc region, larger molecular size, and FcRn-mediated recycling, exhibit longer circulating half-lives compared to Fc-less bsAbs. Their purification process is more straightforward, with improved solubility and stability. Importantly, IgG-like bsAbs may hold greater clinical therapeutic potential, retaining multiple Fc-mediated effector functions, including ADCC, CDC, and antibody-dependent cellular phagocytosis.
03 Comparison of Non-IgG-like bsAb Fragments:
Non-IgG-like bsAb fragments demonstrate relatively lower pharmacokinetic properties compared to IgG-like bsAbs. However, they possess superior tissue penetration capability, lower immunogenicity, and reduced risk of nonspecific activation of the innate immune system. These bsAbs rely primarily on their antigen-binding capacity to exert diversified functions.
04 Strategies for Prolonging Non-IgG-like Structural Half-Life:
To extend the half-life of non-IgG-like structures while maintaining original biological activity, safety, and low immunogenicity, several strategies can be employed to increase their molecular weight and prolong serum half-life. Specific strategies include: (a) using peptide linkers to form antibody fragment multimers; (b) coupling with other molecules such as human serum albumin, polyethylene glycol (PEG), carbohydrates, N-(2-hydroxypropyl) methacrylamide (HPMA), and dextran. Among these, fragment multimerization, particularly using multivalent single-chain antibodies, represents a core strategy for non-IgG-like forms.
Bispecific Antibody Characterization
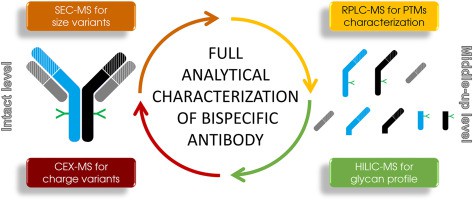 Figure 2. Bispecific Antibody Characterization methods
Figure 2. Bispecific Antibody Characterization methodsCharge Variants in Bispecific Antibodies
Charge variant analysis is crucial in the characterization of bsAbs as it provides insight into the heterogeneity and PTMs that can influence the efficacy, stability, and safety of therapeutic antibodies.
Identification of Post-Translational Modifications
Charge variants arise from various PTMs. Acidic species can result from deamidation, glycation, or glycan modifications, while basic species may be due to C-terminal Lys, Asp isomerization, or Met oxidation. The formation of pyroglutamate (pE) at the N-terminus, depending on whether the precursor is Glu (E) or Gln (Q), also impacts charge. Our CEX-MS analysis identified these PTMs, confirming the presence of high-mannose and afucosylated glycoforms in the acidic variant and complex, fucosylated glycoforms in the basic variants. This detailed PTM profiling is crucial for understanding the biophysical properties of bsAbs.
Homogeneity and Aggregation Analysis
Homogeneity and aggregation analysis are vital components in the characterization of bispecific antibodies. Techniques like SEC-MS, complemented by IEX and HILIC, provide comprehensive data that ensure the stability and efficacy of the antibody product. At Creative Proteomics, the integration of these advanced analytical methods underscores our commitment to delivering high-quality biopharmaceuticals with consistent performance and safety profiles. This meticulous approach to antibody characterization exemplifies our dedication to advancing therapeutic development and ensuring patient safety.
Intact Protein Analysis
Reverse-phase liquid chromatography coupled with mass spectrometry (RPLC-MS) is widely used for intact mass determination. Our intact analysis of bsAbs using both RPLC and HILIC modes revealed better peak shape in RPLC.
| The Category of Characterization Analysis | Items | Methods |
| Primary structure analysis | Complete molecular weight or reduction/desugar molecular weight detection | MALDI-TOF MS / ESI-TOF MS |
| N-terminal sequencing | LC-MS/MS | |
| C-terminal sequencing | LC-MS/MS | |
| Disulfide bond analysis | LC-MS/MS | |
| Sequence coverage analysis | LC-MS/MS | |
| Protein post-translational modification analysis | LC-MS/MS | |
| Glycosylation analysis | Glycosylation site analysis | LC-MS/MS |
| Glycotype analysis and content detection | LC-FLR-MS | |
| Binding activity analysis | Protein binding/inhibition analysis | ELISA(EC50,IC50) |
| Cell binding/inhibition analysis | FACS(EC50、IC50) | |
| Surface Plasmon Resonance (SPR) affinity assay | SPR | |
| Purity analysis | Monomer and polymer purity analysis | SEC-HPLC |
| Non-glycosylated heavy chain and small molecule fragments | SEC-HPLC / SDS-PAGE / CE-SDS | |
| Charge heterogeneity analysis | Charge isomer distribution | IEX-HPLC |
| Distribution of pI and different pI components | CE-IEF / cIEF | |
| Stability analysis | Determination of denaturation temperature | DSF |
At Creative Proteomics, we have developed a comprehensive LC/MS workflow for the detailed characterization of bispecific antibodies. Our integrated approach, combining CEX-MS, SEC-MS, RPLC-MS, and HILIC-MS, ensures thorough analysis of charge variants, size variants, glycosylation profiles, and PTMs. This robust analytical platform not only confirms the correct hetero-dimerization and structural integrity of bsAbs but also provides in-depth insights into their biophysical properties. These methodologies are indispensable for the development and quality control of next-generation therapeutic antibodies, ensuring their efficacy and safety in clinical applications.
References
- Bethany H. James et al,. The Contribution of Liver Sinusoidal Endothelial Cells to Clearance of Therapeutic Antibody. Gastrointestinal Sciences 2022
- Labrijn, A.F., Janmaat, M.L., Reichert, J.M. et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 18, 585–608 (2019).














