As of January 2023, fifteen ADC drugs have been approved for marketing, with over eighty ADC molecules entering clinical trials. ADCs have entered a phase of rapid development, emerging as one of the most promising fields today.
Comprising antibodies, linkers, and drugs, antibody-drug conjugates (ADCs) guide potent small-molecule drugs to tumor cells by binding to surface antigen specificity.
The proper design or selection of antibodies, linkers, and drugs is crucial to achieving the desired therapeutic effect while minimizing off-target toxicity.
ADCs have undergone extensive technological accumulation over the past century, particularly in the last five years, with significant advancements resulting in the emergence of many effective and reliable drugs. Although first and second-generation ADCs have not garnered significant attention in research due to narrow therapeutic windows and significant side effects, the market introduction of third-generation ADCs such as Enhertu and Trodelvy has sparked renewed enthusiasm for ADCs.
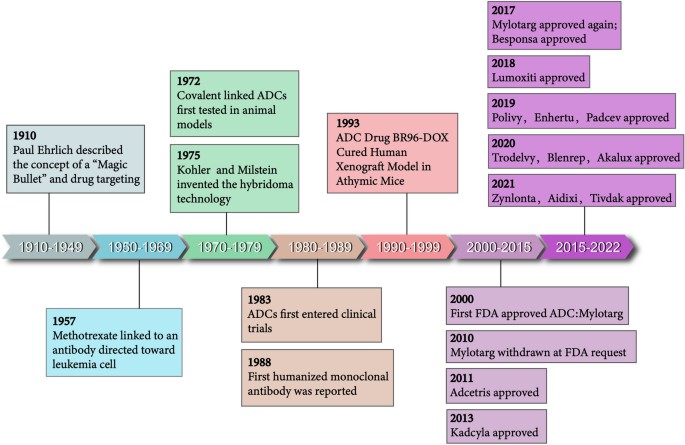 A brief history and key events in ADC drug development
A brief history and key events in ADC drug developmentIn 2022, the FDA approved Enhertu for the treatment of inoperable or metastatic HER2-low breast cancer. Representing the third generation of ADCs, Enhertu holds promise in enhancing ADC therapy efficacy and refining targeted treatment strategies based on ADCs, through optimizations in ADC antigen selection, development of novel cytotoxic drugs, introduction of new linker and conjugation technologies, among other approaches.
As of January 2023, fifteen ADC drugs have been approved for marketing, with over eighty ADC molecules entering clinical trials. ADCs have entered a phase of rapid development, emerging as one of the most promising fields today.
You may interested in
Learn more
Key Elements in ADC Molecular Design
As mentioned earlier, ADCs consist of antibodies, linkers, and toxins. The production of ADC drugs requires these components to be produced separately and then assembled, involving multiple steps with high demands on molecular design, preparation, and manufacturing processes.
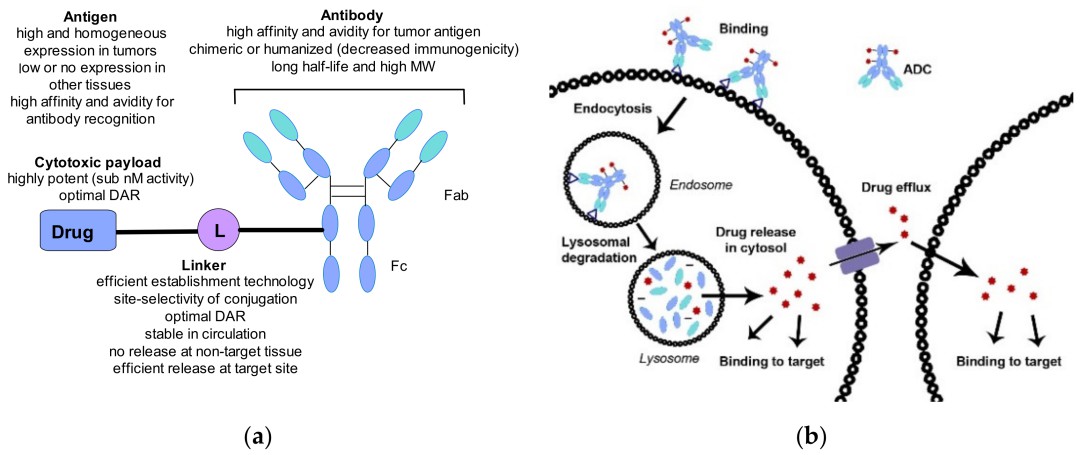 General structure of an ADC and key considerations when combining different components
General structure of an ADC and key considerations when combining different componentsAntigen Targets for ADCs
Expression and Abundance: Antigen targets exhibit high expression levels in tumor tissues and low or absent expression in normal tissues. Ideally, target antigens should be non-secretory, resistant to shedding from the surface of tumor cells, and exist in soluble forms to a lesser extent. The emphasis lies on expression ratios rather than absolute levels, as downregulation post-treatment should be avoided to prevent ADC resistance.
Internalization Properties: The rate and extent of antigen internalization are crucial parameters directly impacting tumor cell uptake and release of ADCs. Additionally, non-internalizing ADCs can still exert therapeutic effects through the "bystander effect" of cytotoxic small molecules.
Antigen Co-Expression and Synergistic Effects: ADCs may encounter challenges such as low tumor antigen expression, limited therapeutic responses, and concerns regarding on-target off-tumor toxicity. Bispecific ADCs combine the advantages of bispecific antibodies with ADCs, offering novel solutions. Bispecific antibody ADCs can precisely target tumor cells, theoretically reducing on-target off-tumor toxicity and improving the therapeutic window. Furthermore, by promoting synergistic interactions between two antigens, such as cross-linking and enhanced internalization, the expression of receptor proteins on the cell membrane can be reduced, thus effectively killing tumor cells and enhancing the efficiency of toxin entry into cells.
Antibodies in ADCs
Specificity and Affinity: Ideal antibodies should possess high specificity, while moderate affinity contributes to increased permeability of ADCs at tumor sites. Proper adjustment of affinity between antigens and antibodies is crucial to balance the rapid absorption of ADCs into target cells and their anticancer effects.
Internalization Efficiency: After binding with target antigens, most ADCs effectively achieve internalization through antibody-mediated endocytosis. Therefore, selecting antibodies with efficient and rapid endocytic activity is important. Some studies are investigating the targeting potential of non-internalizing ADCs towards components of the tumor microenvironment to overcome penetration barriers in solid tumors.
Molecular Type and Fc Functionality: Currently, widely used ADC drugs primarily consist of IgG1 subtype. Partial loss of ADC's Fc effector function is beneficial. Binding of ADC drugs to effector cells may reduce accumulation at tumor sites, hinder their entry into cells, or increase toxicity to normal cells.
In addition to full-length monoclonal antibodies, the antibody portion of ADCs also includes bispecific antibodies and different forms of small molecule antibodies.
Bispecific ADCs offer higher tumor targeting specificity and increased safety. To enhance tumor penetration of ADCs, novel ADC molecules adopt different small molecule-antibody formats such as Fab-drug conjugates, scFv-drug conjugates, and single-chain antibodies (VHH) conjugated drugs. These novel small molecule antibody ADCs have smaller molecular weights and better tumor penetration compared to traditional ADCs but may have shorter half-lives and faster clearance.
Drug Product and Pharmacokinetic Characteristics: After conjugation with toxic small molecules, the properties of ADC molecules may change due to the strong hydrophobicity of small molecule toxins, thus requiring antibodies with better drug-like properties. The next generation of ADCs increasingly utilizes fully humanized antibodies with significantly reduced immunogenicity. ADCs exhibit pharmacokinetic characteristics similar to antibodies. Therefore, ideal antibody selection should also consider low immunogenicity while maintaining optimal PK properties.
The Linker in ADCs
The conjugation linker, an essential constituent in ADCs, plays a pivotal role in bridging antibodies with potent small molecules, thereby influencing the drug's stability, safety, and efficacy. It is imperative for the linker to exhibit robustness in the systemic circulation, while efficiently releasing the drug payload upon encountering tumor cells. Classifications of linkers predominantly comprise cleavable and non-cleavable varieties, with cleavable linkers further delineated into chemical and enzymatic cleavage categories.
Non-cleavable linkers exhibit good circulatory stability, reducing off-target toxicity, but they require lysosomes within tumor cells to release the drug payload, resulting in slower release rates. The drug payload typically comprises an amino acid-linker-effector format. However, due to their poor permeability, their bystander effect induction capability is limited. The most representative example is N-succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC), found in trastuzumab emtansine, commonly known as T-DM1.
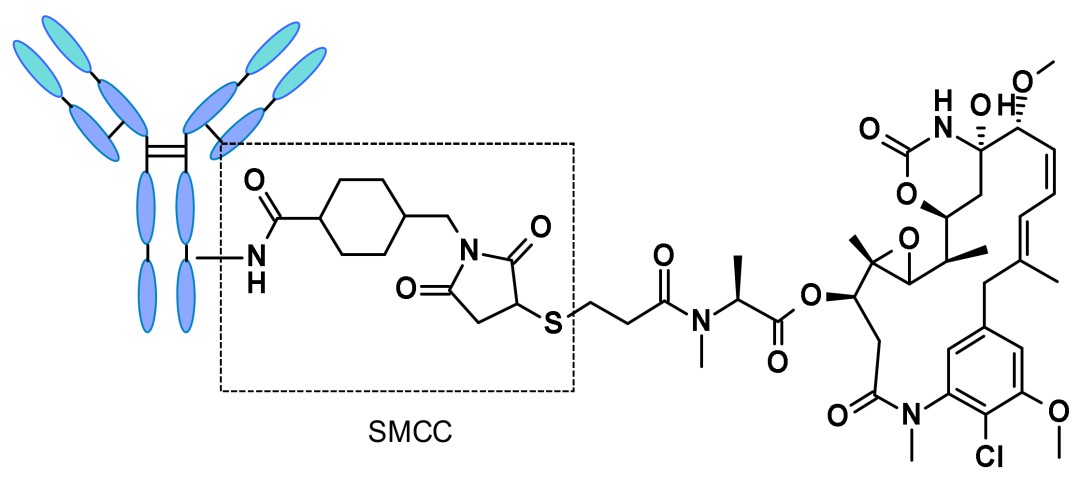 Structure of trastuzumab emtansine (Kadcyla)
Structure of trastuzumab emtansine (Kadcyla)Chemical cleavage linkers primarily consist of acid-cleavable and reduction-cleavable types. These linkers can rapidly release drugs upon cleavage but exhibit poor circulatory stability, which may lead to off-target toxicity.
Among them, enzymatic cleavage linkers are the most widely applied and researched type, including tissue protease B, β-glucuronidase, and sulfatase cleavage types. Among these, tissue protease B cleavage type is the most commonly used. Tissue protease B is a lysosomal protease overexpressed in various cancer cells. Moreover, it selectively recognizes specific amino acid sequences such as PL, VC, VA, and GGFG, cleaving the peptide bonds at the C-terminus of these sequences. This type of linker combines good circulatory stability with rapid cleavage and toxin release within tumor cells.
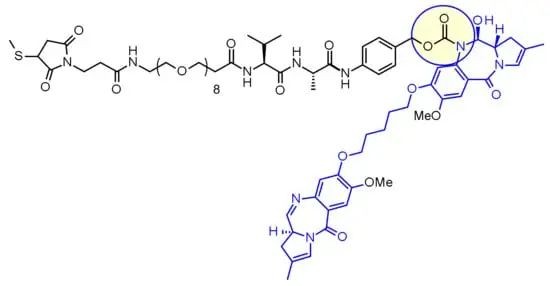 Chemical structure of Loncastuximab tesirine
Chemical structure of Loncastuximab tesirineCytotoxic Payloads in ADCs
The cytotoxic payload in ADCs is crucial for their efficacy. Traditional cytotoxic payloads typically include microtubule inhibitors and DNA-damaging agents, known for their potent activity, good water solubility, and stability. While targeting tumor cells, it is imperative to minimize damage to normal cells. Besides traditional payloads, numerous innovative payloads are under research and development.
Microtubule-targeting toxins primarily function by binding to tubulin, thereby impeding microtubule polymerization or inducing continuous elongation, disrupting cell division. Common examples include auristatins, maytansinoids, and auristatin E derivatives (MMAE), particularly renowned for their activity and penetration.
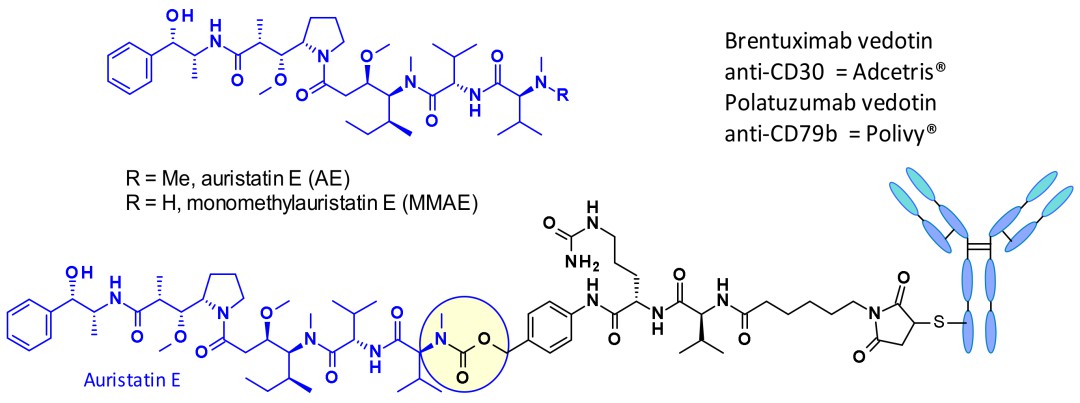 Structure of auristatin E (AE), monomethyl auristatin E (MMAE), and commercially approved auristatin-based ADCs
Structure of auristatin E (AE), monomethyl auristatin E (MMAE), and commercially approved auristatin-based ADCsDNA-damaging toxins primarily consist of DNA fragmenting agents, DNA alkylating agents, and topoisomerase I/II inhibitors. They are not influenced by the cell cycle, exhibiting short half-lives and broad anticancer effects. Common examples include PBD, doxorubicin, mitomycin C, SN38, and DXd, among which DXd stands out as one of the most potent toxins.
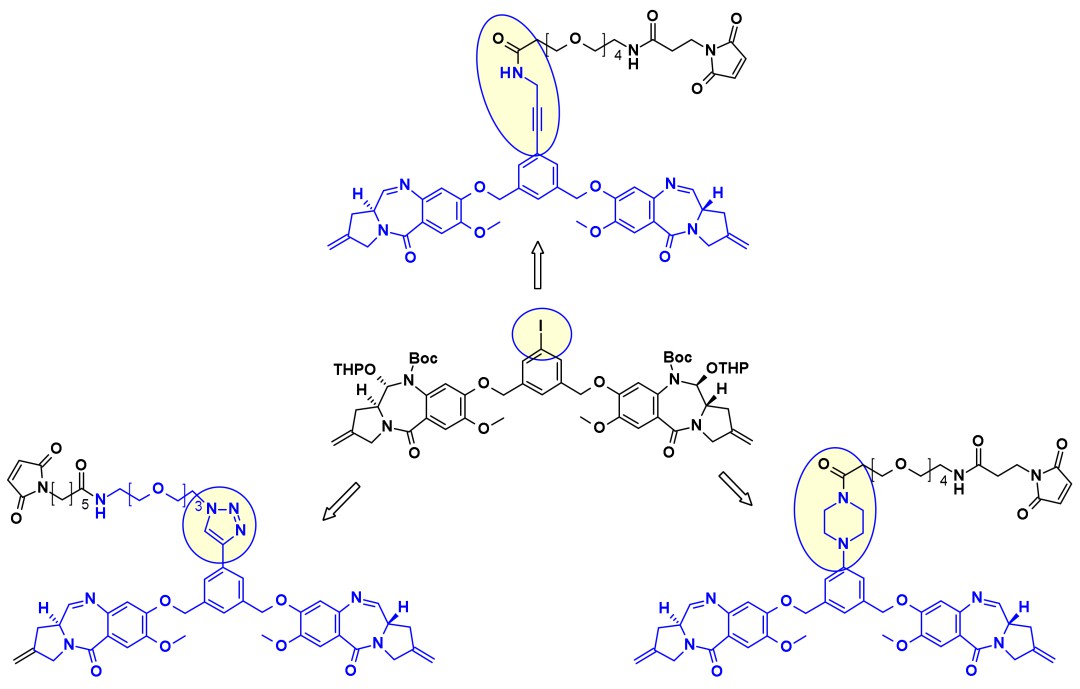 Conjugate PBD dimers/linkers using common iodobenzene intermediates
Conjugate PBD dimers/linkers using common iodobenzene intermediatesIn addition to traditional payloads, innovative payloads such as TLR7/8 agonists, STING agonists, Bcl-xL inhibitors, and clastosome inhibitors have emerged.
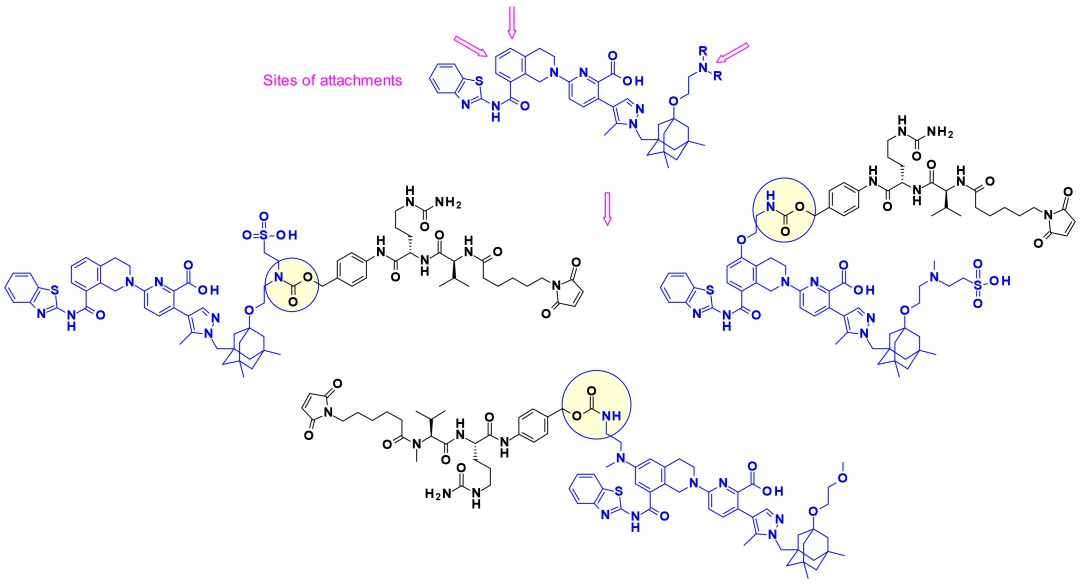 Attachment sites on typical Bcl-xL inhibitors and some reported payload-linker conjugates
Attachment sites on typical Bcl-xL inhibitors and some reported payload-linker conjugatesConjugation techniques play a crucial role in the homogeneity, stability, and pharmacokinetics of ADCs, classified into non-site-specific conjugation and site-specific conjugation. Non-site-specific conjugation includes lysine conjugation and random cysteine conjugation. While lysine conjugation methods exhibit poor selectivity, random cysteine conjugation significantly enhances stability and homogeneity. Site-specific conjugation comprises cysteine site-specific conjugation, natural antibody site-specific conjugation, and antibody site-directed engineering modifications. Site-specific conjugation techniques achieve homogeneity and stability, expanding the therapeutic window. This includes saturation conjugation, Thiomab, and bridging methods, as well as lysine, glycan modification, and non-natural amino acid conjugation techniques.
Current Challenges in ADCs Design
Complexity of Drug-to-Antibody Ratio (DAR): DAR, the ratio of drugs to antibodies, stands as a critical parameter in ADC design, directly influencing drug payload and distribution. High DAR values may induce product instability and aggregate formation, thereby diminishing efficacy and increasing toxicity.
Complexity of Pharmacokinetics: Pharmacokinetic properties of ADCs are influenced by various factors, including DAR values, antibody structure, and drug release rates. ADCs with different DAR values may exhibit diverse in vivo behaviors, posing challenges in predicting and controlling clinical efficacy.
Immunogenicity: Components of ADCs may elicit immune reactions, such as the production of anti-drug antibodies (ADAs), thereby affecting therapeutic efficacy and safety. Consideration of immunogenicity in ADC design and measures to mitigate its risks are necessary.
Resistance: Resistance of tumor cells to ADCs is a common issue in therapy. Mechanisms of resistance include downregulation of target antigen expression, high expression of drug efflux transporters, and defects in lysosomal function. Comprehensive understanding and addressing of these mechanisms are crucial for enhancing ADC therapeutic efficacy.
Therapeutic Window: Broadening the therapeutic window of ADCs is crucial for improving their efficacy. This involves strategies such as optimizing target selection, antibody structure, drug payload, linker design, conjugation techniques, and pharmacokinetics to achieve higher efficacy and lower toxicity.
References
- Li, Meng, et al. "Antibody-Drug Conjugate Overview: a State-of-the-art Manufacturing Process and Control Strategy." Pharmaceutical Research (2024): 1-22.
- Kostova, Vesela, et al. "The chemistry behind ADCs." Pharmaceuticals 14.5 (2021): 442.












