Membrane transport proteins represent a pivotal group of proteins within physiology and pathological contexts. Within pharmacokinetic considerations, it is widely acknowledged that membrane transport proteins play a critical role in the absorption, distribution, and excretion of drugs, thereby regulating the membrane transport of pharmaceutical agents.
What are Membrane Transport Proteins
Membrane transport proteins, a subclass of membrane proteins, are proteins present on biological cell membranes, primarily tasked with facilitating the transportation of various molecules between intracellular and extracellular compartments. These molecules encompass pharmaceutical agents, metabolites, nutrients, ions, among others. By modulating the transport of these molecules, membrane transport proteins significantly influence their absorption, distribution, metabolism, and excretion within organisms.
The Role of Membrane Transport Proteins in Drug Research and Clinical Practice: Membrane transport proteins hold significance in drug research and clinical practice due to their impact on the absorption rate, tissue distribution, metabolic rate, and excretion rate of pharmaceuticals. Additionally, these proteins serve as pivotal factors in drug interactions and adverse drug reactions, potentially affecting drug concentrations and duration of action within the body. Common membrane transport proteins include ATP-binding cassette transporters (ABC transporters) and solute carrier transporters (SLC transporters), which are responsible for transporting different types of molecules. By investigating the structure and function of membrane transport proteins, scientists can gain a better understanding of drug behavior within the body, thereby guiding drug design and therapeutic strategies.
Major Membrane Transport Proteins include:
ATP-binding cassette transporters (ABC transporters): This extensive class of membrane transport proteins utilizes ATP (adenosine triphosphate) as an energy source to facilitate substance transport across cell membranes. Among them, ABCB1 (also known as P-glycoprotein or MDR1) serves as a representative, extensively expressed in tissues such as the intestines, liver, and kidneys, participating in drug absorption and excretion.
Organic anion transporting polypeptides (OATP transporters): Represented by OATP1B1 and OATP1B3, these proteins are widely expressed in the liver, participating in the transport of organic anions, including certain drugs and metabolites.
Organic anion transporters (OAT transporters) and organic cation transporters (OCT transporters): These two transporter classes are respectively responsible for the transport of organic anions and organic cations, participating in the excretion and absorption of drugs in the kidneys, liver, and other tissues.
Multidrug resistance-associated proteins (MRP transporters): Represented by MRP1, MRP2, among others, these transporters participate in multidrug resistance and are extensively expressed in tissues such as the liver and kidneys, facilitating the excretion of drugs and metabolites.
These membrane transport proteins play crucial roles in maintaining intracellular and extracellular substance balance, drug metabolism, and toxin clearance, thereby exerting influence on drug absorption, distribution, metabolism, and excretion processes.
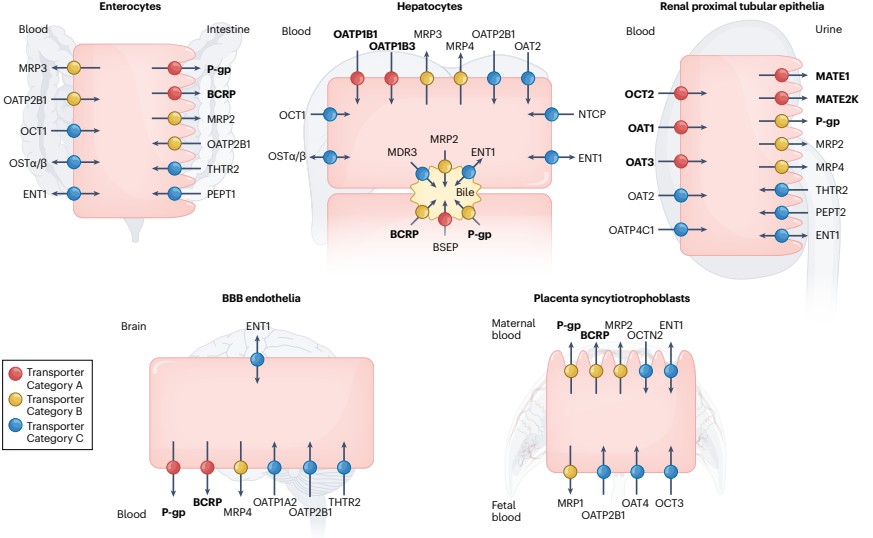 Clinically important uptake and efflux transporters in plasma membranes
Clinically important uptake and efflux transporters in plasma membranesInvestigating Membrane Transport Proteins
The expression patterns of membrane transport proteins during human development have become a focal point of scientific investigation. To delve into the functionality and localization of these proteins within cells and tissues, researchers employ a spectrum of highly specialized techniques. In addition to conventional immunohistochemical methods for visualizing protein localization within tissues, more precise and sensitive techniques are utilized to quantify the expression levels of transport proteins.
Reverse transcription polymerase chain reaction (RT-PCR):
Among these, reverse transcription polymerase chain reaction (RT-PCR) stands as a widely applied method for analyzing messenger RNA (mRNA) expression levels. Through RT-PCR, researchers can quantitatively assess the transcription levels of membrane transport proteins across different tissues and developmental stages, thereby elucidating their regulatory mechanisms within organisms.
Western blotting:
Furthermore, Western blotting techniques are extensively employed to detect the presence and expression levels of proteins. By separating proteins from samples and identifying them using specific antibodies, researchers can accurately quantify the protein levels of transport proteins, thereby revealing their abundance and regulation within cells.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology:
Recently, the development of liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology has provided a novel perspective on membrane transport protein research. This high-throughput technique enables precise protein quantification with high specificity and sensitivity. Through LC-MS/MS, researchers can simultaneously analyze large numbers of protein samples and quantitatively measure the abundance of membrane transport proteins, thereby gaining a more comprehensive understanding of their roles in cellular function and biological processes.
You may interested in
Investigation of Pharmacokinetics and Pharmacogenetics of Membrane Transport Protein Substrates
The study of pharmacokinetics and pharmacogenetics of membrane transport protein substrates refers to the scientific domain investigating the membrane transport proteins that influence the absorption, distribution, metabolism, and excretion of drugs within the human body. These proteins are located on cell membranes and regulate the pharmacokinetics of drugs by modulating the transport of drug molecules both intra- and extracellularly, thus impacting the efficacy and safety of medications.
Research into the pharmacokinetics and pharmacogenetics of membrane transport protein substrates holds significant importance. Firstly, understanding the role of membrane transport proteins in drug transport and metabolism within the human body aids in optimizing drug therapy regimens, thereby enhancing drug efficacy and reducing adverse reactions. Secondly, pharmacogenetic studies can unveil how individual genetic variations affect the functionality of membrane transport proteins, consequently influencing the pharmacokinetics and clinical effects of drugs. This provides a theoretical basis for personalized drug therapy, enabling physicians to select the most appropriate drug doses and treatment regimens based on patients' genetic backgrounds and drug metabolism characteristics.
Currently, the clinical focus is on several transport proteins including ABCB1, ABCC2, OATP1B1, and OATP1B3. Studies have revealed that the developmental patterns of these transport proteins may vary across different organs. For instance, ABCB1 can be detected in fetal intestinal samples, while its expression in the liver gradually increases to adult levels after birth. Similarly, the expression pattern of ABCC2 resembles that of ABCB1, but its expression levels in the liver are significantly lower than in adults. As for OATP1B1 and OATP1B3, their expression in the liver exhibits different developmental patterns, with lower expression levels in neonates and infants, stabilizing in adults. These studies, through meticulous observation of the expression levels of transport proteins across different age groups and tissues, provide crucial insights into human drug metabolism and disposition.
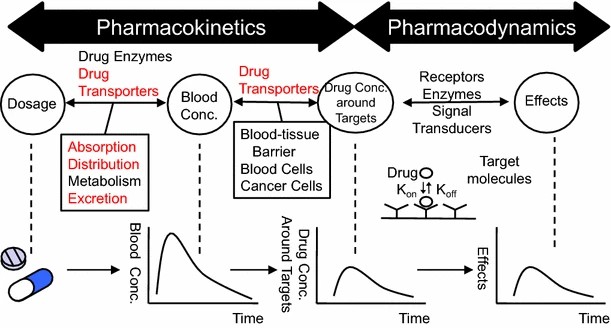 PK/PD relationships and drug transporters
PK/PD relationships and drug transportersIn the realm of technological advancement, the investigation of membrane transporter protein substrates' pharmacokinetics and pharmacogenetics typically entails the utilization of both in vitro and in vivo experimental methodologies. In vitro experiments are commonly employed to assess the transport and metabolism of drugs by membrane transporter proteins, encompassing cell culture techniques and membrane vesicle assays. Conversely, in vivo experiments typically encompass clinical pharmacokinetic studies and pharmacogenetic investigations, involving the collection of human specimens for drug concentration determination and genetic analysis to evaluate the role of membrane transporter proteins in individual drug metabolism. By harnessing these methodologies, researchers are empowered to comprehensively elucidate the impact of membrane transporter proteins on drug metabolism, thereby furnishing pivotal insights for personalized drug therapy.
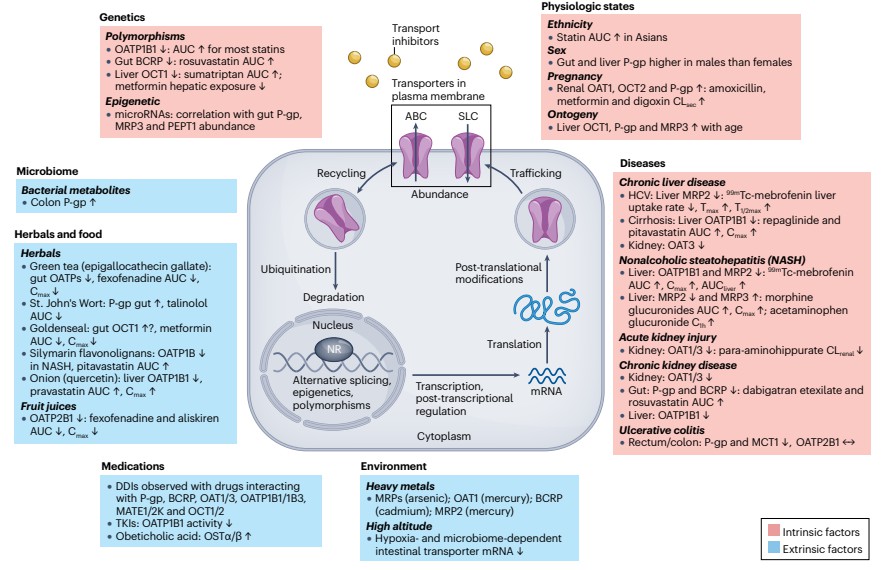 Intrinsic and extrinsic factors that influence the abundance and/or activity of drug transporters and the mechanisms that may be involved
Intrinsic and extrinsic factors that influence the abundance and/or activity of drug transporters and the mechanisms that may be involvedTable 1. Partial Membrane Transport Proteins Associated with Drug Substrates
| Drug | Associated Transport Proteins |
| Digoxin | ABCB1 |
| Tacrolimus | ABCB1 |
| Daptomycin | ABCB1 |
| Fexofenadine | OATP2B1, ABCB1, MRP2 |
| Morphine | OCT1, ABCB1, ABCC2, ABCC3, OATP1B1 |
| Pravastatin | OATP1B1, OATP2B1, OATP1B3, ABCB1, ABCC2 |
| Atorvastatin | OATP1B1, BCRP |
| Bosentan | OATP1B1, OATP1B3, OATP2B1 |
| Ondansetron | OCT1 |
| Metformin | OCT, MATE1, MATE2 K |
| Cimetidine | OCT2, MATE1, MATE2 K, OAT2 |
| Tramadol | OCT1 |
| Methotrexate | OATP1B1, ABCC2 |
| Mycophenolate mofetil | MRP2 |
| Acyclovir/valacyclovir | 4F2hc, HPT1, OAT1, OAT3 |
| Adefovir | OAT1, MRP4 |
Digoxin - ABCB1
Digoxin is a well-known substrate of ABC transport proteins, with its pharmacokinetics being influenced by inducers or inhibitors of intestinal or renal ABC transport proteins. The drug is primarily eliminated via renal clearance, with 80% undergoing glomerular filtration and 20% undergoing tubular secretion. Pharmacokinetic studies in children indicate that the clearance of orally administered digoxin exhibits nonlinear increases with increasing body weight and gestational age. Specifically, in neonates, the clearance of orally administered digoxin increases with increasing body weight, yet, compared to body weight, the clearance of digoxin appears to be higher in full-term neonates and infants. However, due to immature glomerular filtration, premature infants exhibit lower digoxin clearance rates. The clearance of digoxin in children may be influenced by intestinal and renal ABC transport proteins, especially in the presence of the ABC transport protein inhibitor, carvedilol. Although murine studies support the hypothesis of higher renal expression of ABC transport proteins postnatally, data in human neonates are insufficient to substantiate this viewpoint.
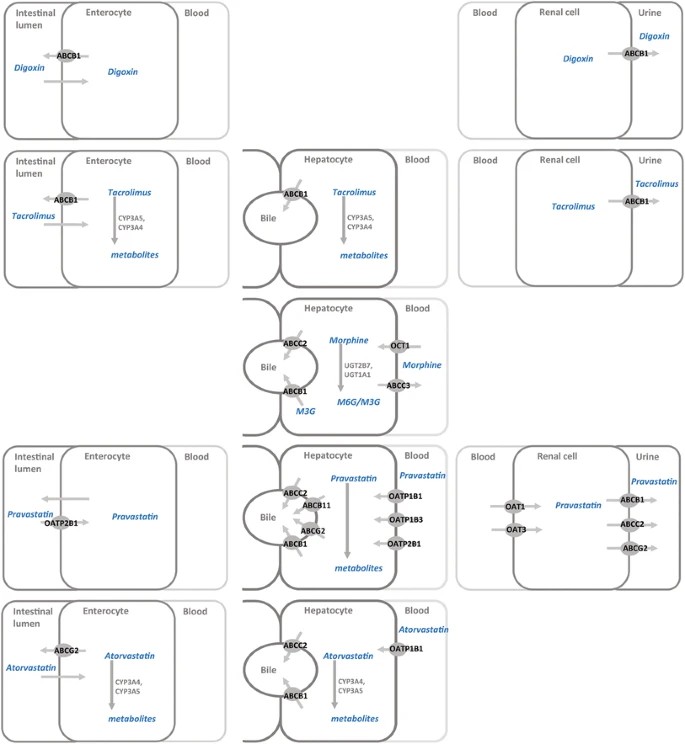 Membrane transport proteins and their relationship to commonly used medications in children: digoxin, tacrolimus, morphine, pravastatin, and atorvastatin.
Membrane transport proteins and their relationship to commonly used medications in children: digoxin, tacrolimus, morphine, pravastatin, and atorvastatin.Tacrolimus - ABCB1
Tacrolimus is a calcineurin inhibitor used for preventing transplant rejection by inhibiting the synthesis of cytotoxic lymphocytes. In vivo, tacrolimus undergoes metabolism primarily via ABCB1 transport proteins in the intestines and liver. Studies reveal that the clearance rate of orally administered tacrolimus is higher during infancy, contingent upon the metabolism by CYP3A4/5 enzymes, which mature within the first year of life. Concurrently, there exist certain contradictions in pharmacogenetic studies associating ABCB1 with tacrolimus. In some pediatric transplant recipients, no correlation was found between ABCB1 genotypes and tacrolimus dose requirements or drug concentration/dose ratios. However, among some liver transplant patients, homozygous ABCB1 1236TT/2677TT/3435TT genotypes necessitated higher tacrolimus doses post-transplantation. Conversely, in certain pediatric liver transplant recipients, the ABCB1 2677G>T allele was associated with higher pre-dose and concentration/dose ratios on the first day post-transplantation. These findings may partially elucidate in vitro findings suggesting stable intestinal ABCB1 mRNA expression but lower hepatic ABCB1 expression in neonates. These results suggest the influence of intestinal ABCB1 on tacrolimus metabolism, especially in situations where intestinal ABCB1 expression levels increase post-transplantation.
Daptomycin - ABCB1
Daptomycin is an antibacterial agent used to treat Gram-positive bacterial infections, including methicillin-resistant Staphylococcus aureus (MRSA). It is primarily excreted via the kidneys and is also a substrate of ABCB1 transport proteins. Studies indicate that certain variations in the ABCB1 gene, such as the 3435T single nucleotide polymorphism, are associated with increased plasma drug concentrations and decreased clearance rates of intravenous daptomycin in adult Caucasian patients. In children, smaller infants exhibit higher daptomycin clearance rates, while clearance rates are comparable between premature and full-term infants. This pharmacokinetic pattern, akin to that of digoxin, with its high clearance rates, may be linked to the role of ABCB1 transport proteins in the renal tubules during infancy. Further research is warranted to corroborate these findings.
Fexofenadine - OATP2B1
Fexofenadine is an antihistamine drug primarily excreted via bile and undergoes minimal metabolism by intestinal microbiota and CYP3A4. Its metabolism is influenced by the uptake transporter OATP2B1, efflux transporter ABCB1, and possibly ABCC2. Some studies suggest that the clearance of fexofenadine in healthy men is associated with variations in OATP2B1 and simultaneous intake of apple juice. This finding of food-drug interaction may be more pertinent in children, as they may consume more apple juice. Pharmacokinetic studies have revealed variations in fexofenadine clearance rates among different age groups. For instance, higher clearance rates were observed in Japanese children aged 6 to 12 years compared to those under 1 year old. Such differences also exist among different ethnicities, although this may be due to small sample sizes rather than racial disparities. Although lacking in in vitro data, gene expression analysis in neonatal intestinal samples obtained during surgeries revealed OATP2B1 gene expression levels nearly three times higher than in adults. This suggests potentially increased oral absorption of fexofenadine in neonates and small infants.
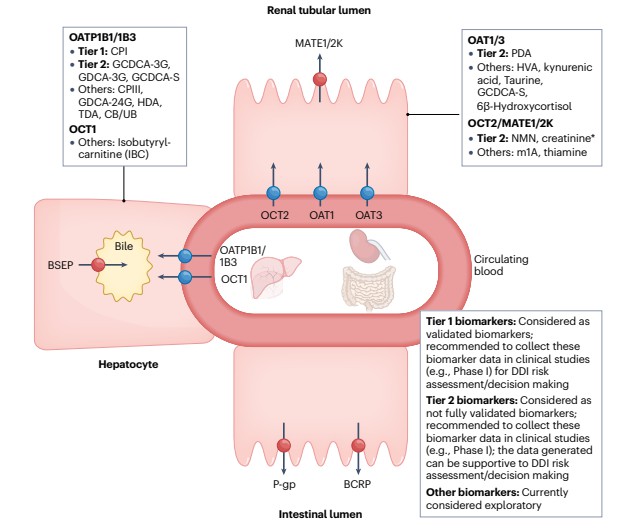 Classification of endogenous biomarkers of liver and kidney transporters and recommendations from the International Transporter Consortium for their application in drug development
Classification of endogenous biomarkers of liver and kidney transporters and recommendations from the International Transporter Consortium for their application in drug developmentPravastatin - OATP1B1
Pravastatin, a cholesterol synthesis inhibitor, undergoes primarily non-CYP450-mediated drug metabolism and interactions with various transport proteins, including OATP1B1, OATP2B1, OATP1B3, ABCB1, and ABCC2. In adults, genetic variations in the SLCO1B1 gene have been linked to differences in pravastatin pharmacokinetics, highlighting the significant role of SLCO1B1 in pravastatin metabolism.
A recent pharmacogenetic analysis involving children with hypercholesterolemia revealed that those with the SLCO1B1-11187GA genotype experienced lower exposure to pravastatin compared to their wild-type counterparts. Interestingly, findings from adult counterparts yielded contrasting results. However, given the limited sample size, these results warrant careful consideration, though they do suggest potential variations in OATP1B1 expression concerning age.
Despite these observations, pravastatin pharmacokinetics in children appear to mirror those in adults, indicative of a consistent impact of transport proteins, like OATP1B1, on pravastatin metabolism throughout different ages. This consistency aligns with the sustained expression of OATP1B1 in the livers of older children, suggesting age-related changes in the activity of these transport proteins may not significantly affect pravastatin levels beyond five years of age.
Methotrexate - OATP1B1 and ABCC2
Methotrexate, used for cancer treatment and anti-inflammatory purposes, undergoes complex hepatic and intracellular metabolism. It is primarily eliminated via renal excretion, with several membrane transport proteins responsible for its uptake and excretion. The clearance rate of methotrexate in children decreases slightly in the first few months after birth but gradually increases thereafter until linear decline begins after one year of age. Pharmacogenetic studies suggest associations between variations in the SLCO1B1 gene and methotrexate pharmacokinetics and toxicity in pediatric leukemia patients. These studies also identify relationships between the ABCC2 gene and methotrexate pharmacokinetics and toxicity, particularly in specific populations.
References
- Galetin, A., Brouwer, K.L.R., Tweedie, D. et al. Membrane transporters in drug development and as determinants of precision medicine. Nat Rev Drug Discov (2024).
- Guan, L. Structure and mechanism of membrane transporters. Sci Rep 12, 13248 (2022). https://doi.org/10.1038/s41598-022-17524-1
- Mooij, Miriam G., et al. "Development of human membrane transporters: drug disposition and pharmacogenetics." Clinical Pharmacokinetics 55 (2016): 507-524.







