In the development of antibody and protein therapeutics, cell banks represent the foundational starting point for drug production. Their quality directly determines the stability, safety, and overall success of subsequent manufacturing processes. As the cornerstone of quality control for master cell banks (MCBs) and working cell banks (WCBs), cell bank characterization is not only a regulatory requirement but also a critical prerequisite for ensuring the seamless progression of drug development pipelines. However, the specific technical requirements and testing workflows involved in cell bank characterization can often appear intricate and detail-intensive to many research and development teams.
To facilitate a clearer understanding and effective implementation of cell bank characterization, this article systematically analyzes its key aspects from a technical perspective. Beginning with the classification and fundamental requirements of cell banks, we will provide a step-by-step introduction to the core testing parameters involved in the characterization process. These include cell identification, contaminant detection (e.g., bacteria, fungi, mycoplasma, and viral agents), as well as assessments of tumorigenicity and oncogenicity. Collectively, these tests not only ensure the safety and stability of the cell bank but also serve as pivotal components in guaranteeing the regulatory compliance and reliability of antibody and protein drug development.
From comprehensive screening for adventitious agents to precise detection of specific viruses (such as bovine- or porcine-derived viruses), cell bank characterization underpins every critical stage of pharmaceutical production. By gaining a deeper understanding of these characterization requirements, research teams can more effectively mitigate risks and establish a robust foundation for subsequent drug development efforts. The following sections will provide an in-depth exploration of the technical requirements and practical methodologies for cell bank characterization.
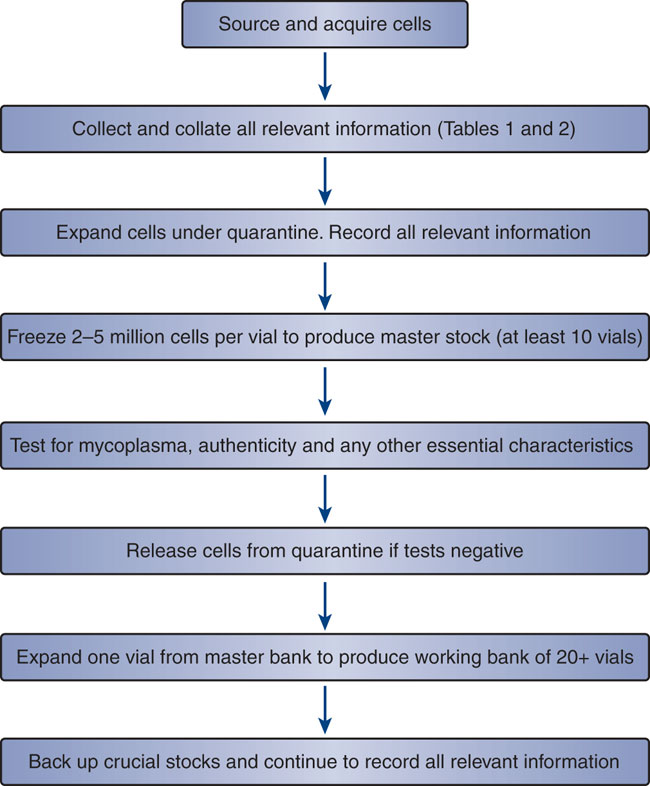 An overview of the stages involved in cell banking. (Glyn Stacey et al,. 2008)
An overview of the stages involved in cell banking. (Glyn Stacey et al,. 2008)Classification of Cell Banks
The management of cell banks typically follows a three-tiered system comprising the pre-master cell bank (also known as the cell seed or MCB, and the WCB.
Pre-Master Cell Bank (Pre-MCB)
Also referred to as the cell seed or primary cell bank (PCB), the pre-master cell bank originates from an initial cell population. This population is expanded into a stable passaged group of cells or a homogeneous clonal population through selective culturing. Upon verification, these cells are deemed suitable for the production or testing of biological products. Under specific conditions, a uniform cell suspension is aliquoted into cryovials or ampoules and cryopreserved at temperatures below −130°C, forming the primary cell bank. This bank serves as the source for establishing the MCB.
MCB
The master cell bank is generated by propagating cells from the pre-master cell bank into a single batch, homogenizing the cells, and cryopreserving aliquots at temperatures below −130°C. These cells undergo comprehensive quality control testing based on specific regulatory requirements. Only after meeting all prescribed standards can the batch be designated as the MCB, which serves as the source for creating the WCB. The quality standards of the MCB are more stringent than those of the pre-master cell bank. Regulatory guidelines mandate that the MCB used in production must not exceed two cell passages.
WCB
The working cell bank is derived from the MCB through further propagation to a defined passage level. The cells are then pooled, homogenized, and cryopreserved in aliquots at temperatures below −130°C. The passage level during cryopreservation must ensure that, after recovery, sufficient proliferation capacity remains to produce at least one batch or sub-batch of the final product. The post-thaw passage level must not exceed the approved upper limit, which is determined based on experimental results and aligned with internationally recognized standards. The quality standards of the WCB are established in accordance with those of the MCB. Each WCB batch must pass stringent testing before being approved for use in production. Additionally, regulatory guidelines specify that a single passage level is permitted for the WCB in a manufacturing setting.
| Type of Cell Bank | Purpose | Key Characteristics |
| Research Cell Bank (RCB) | Used for research and development | Pre-GMP; diverse cell lines; initial source for MCBs |
| Master Cell Bank (MCB) | Primary source for generating WCBs | Well-characterized; cryopreserved; rigorous testing |
| Working Cell Bank (WCB) | Immediate source for production | Derived from MCB; sufficient quantity for ongoing use |
Basic Requirements for Cell Bank Characterization
The extent of cell bank characterization depends on the intended use of the bank and associated risk management principles:
R&D Cell Banks
Research and development (R&D) cell banks are typically established within laboratory environments. As samples derived from these banks are not intended for human trials, extensive characterization is not required. Minimal testing, such as assessments for mycoplasma and sterility, is usually sufficient.
MCB and WCB
MCBs and WCBs, in contrast, must be established under Good Manufacturing Practice (GMP) conditions. These banks are used to produce materials for human trials or other critical experimental studies and must therefore adhere to rigorous safety and quality standards.
Pharmacopoeial Requirements
According to pharmacopoeial guidelines:
- The MCB and end-of-production cells (EOPC) must undergo comprehensive testing at least once after the establishment of the cell bank.
- Any significant changes in the manufacturing process necessitate re-testing of the EOPC.
- Each newly established WCB from the MCB must be tested for specified parameters.
Key Aspects of Cell Bank Characterization
- Cell identification: Verification of cell line identity through techniques such as isoenzyme analysis or genetic profiling.
- Bacterial and fungal testing: Comprehensive sterility assessment to detect bacterial or fungal contamination.
- Mycobacteria testing: Specialized assays to screen for Mycobacterium species.
- Mycoplasma detection: Identification of mycoplasma contamination, which can compromise cell culture integrity.
- Viral factor detection – Morphology and hemadsorption: Observation of cell morphology and hemadsorption assays to detect viral activity.
- Viral factor detection – In vitro assays: Virus detection through inoculation of indicator cell lines.
- Viral factor detection – In vivo assays: Assessment of viral contaminants using animal inoculation methods.
- Viral factor detection – Retroviruses and endogenous viruses: Detection of retroviruses or other endogenous viral elements through molecular or serological methods.
- Viral factor detection – Species-specific viruses: Targeted detection of species-specific adventitious viruses.
- Viral factor detection – Bovine-derived viruses: Screening for viruses of bovine origin.
- Viral factor detection – Porcine-derived viruses: Screening for viruses of porcine origin.
- Viral factor detection – Other specific viruses: Detection of other viruses based on regulatory or research needs.
- Tumorigenicity testing: In vivo assessment of the potential for cells to form tumors.
- Oncogenicity testing: Evaluation of the likelihood that cells can induce tumor formation in host tissues.
Cell Line Identification
Newly established cell lines, cell banks (including MCB and WCB), and end-of-production cells must undergo rigorous identification testing to confirm their authenticity and ensure the absence of cross-contamination with other cell types. Several methods are available for cell identification, each offering distinct advantages depending on the context of the cell line and the specific characteristics to be verified. These methods include:
- Cell morphology: Microscopic examination of cellular characteristics and growth patterns.
- Biochemical methods: Techniques such as isoenzyme analysis to differentiate between cell lines based on their enzyme profiles.
- Immunological assays: Identification using tissue-specific antigens, including histocompatibility antigens and species-specific immune sera.
- Cytogenetic analysis: Chromosomal karyotyping and the detection of marker chromosomes for genetic characterization.
- Genetic markers: Techniques like DNA fingerprinting, including short tandem repeat (STR) profiling, restriction fragment length polymorphism (RFLP-PCR), and intron polymorphism (EPIC-PCR) for precise genetic identification.
- Additional methods: Other molecular approaches such as hybridization, polymerase chain reaction (PCR), and reporter gene assays can also be employed for cell identification.
At least one or more of these methods should be used to confirm both the species and the unique characteristics of the cell line, ensuring its authenticity and purity.
Bacterial and Fungal Testing
To ensure sterility, bacterial and fungal testing is performed on samples from mixed cell culture supernatants or cryopreserved cell vials, in accordance with regulatory requirements. For both the MCB and WCB cultures, a minimum of 10 mL of mixed cell culture supernatant should be collected, with membrane filtration being the preferred method for detection. For cryopreserved cells, at least 1% of the total number of cryovials, or at least two vials (whichever is larger), should be tested using the direct inoculation method.
Membrane Filtration Method
The membrane filtration method involves the use of a closed membrane filter system, with filter materials selected based on the characteristics of the test sample and its solvent. For sterility testing, the membrane pore size should not exceed 0.45 μm, and the membrane diameter should be approximately 50 mm. If filters of different sizes are used, adjustments must be made to the volumes of diluents and wash solutions, and revalidation is required. During testing, it is essential to ensure the integrity of the filter both before and after filtration.
The membrane filtration method is designed to detect a broad spectrum of microorganisms, typically including the following six species:
- Staphylococcus aureus
- Pseudomonas aeruginosa
- Bacillus subtilis
- Clostridium sporogenes
- Clostridium botulinum
- Aspergillus niger
Direct Inoculation Method
The direct inoculation method is applied when the membrane filtration method is not suitable for sterility testing. In this approach, a specified volume of the test sample is inoculated in equal amounts into both thioglycolate broth and tryptic soy broth (TSB). For non-biological samples, the inoculated bottles or vials should be equally divided between the two culture media. In the case of biological products, the ratio of inoculated bottles should be 2:1 between thioglycolate broth and TSB.
Unless otherwise specified, the volume of the culture medium in each container must not exceed 10% of the volume of the test sample. Additionally, the minimum volumes for each medium should be:
- Thioglycolate broth: at least 15 mL per vial
- TSB: at least 10 mL per vial
The volumes and heights of the media should be adjusted as per method suitability testing for the specific type of sample being examined.
Interpretation of Results
Sterility is confirmed if all test vials remain clear or, in cases where slight turbidity is observed, if no microbial growth is confirmed upon further examination. If the sample meets these criteria, it is deemed to comply with the required standards.
Mycobacteria Testing
To detect the presence of mycobacteria, prepare a cell lysate from at least 10^7 viable cells using the culture supernatant. The mycobacteria testing should be conducted in accordance with sterility testing procedures.
Inoculation Procedure: Inoculate the cell lysate onto an appropriate solid medium, such as Roche medium or Middlebrook 7H10 agar. For each medium, inoculate 1 mL of the sample, with three replicates performed per sample. Additionally, use a positive control consisting of a Mycobacterium phlei suspension, ensuring that the inoculum does not exceed 100 CFU.
Incubation: Incubate the inoculated media at 37°C for 56 days. The positive control should exhibit visible growth of mycobacteria, while the test sample should show no growth of mycobacteria.
Interpretation: If no mycobacterial growth is observed on the test cultures and the positive control yields growth, the test sample is considered sterile and meets the required specifications.
Mycoplasma Testing
Mycoplasma testing should be performed on MCB, WCB, virus seed lots, control cells, and cells intended for clinical use. Both culture methods and indicator cell culture methods (e.g., DNA staining) should be used simultaneously. For virus vaccines, mycoplasma testing of the viral harvest or raw material is performed using culture methods. In certain cases, the indicator cell culture method can be employed to screen the culture medium. Alternatively, other methods recognized by the national drug inspection authorities may also be used. Cell culture supernatant samples should be tested in compliance with regulatory standards.
Culture Method
Mycoplasma detection is typically conducted using either liquid or semi-solid mycoplasma culture media, or mycoplasma agar media. The semi-solid or agar media should be boiled for 10-15 minutes, cooled to approximately 56°C, and supplemented with inactivated fetal bovine serum (culture medium: serum ratio of 8:2). Penicillin may be added as needed. For liquid media, no boiling is necessary, but the same components should be added before use.
For testing, prepare four vials of mycoplasma liquid culture medium (10 mL per vial) and two vials of semi-solid or agar medium (pre-cooled to 36°C ± 1°C). Inoculate 0.5–1.0 mL of the test sample into each vial of culture medium, then incubate at 36°C ± 1°C for 21 days. On the 7th day after inoculation, subculture 2 vials from each of the 4 liquid culture media to generate secondary cultures. Transfer each culture to both semi-solid and liquid mycoplasma media (2 vials each) and incubate at 36°C ± 1°C for 21 days, observing the cultures every 3 days.
Result Interpretation: At the end of the incubation period, if no mycoplasma growth is observed in any of the test cultures, the sample is deemed sterile and compliant. If there is suspicion of mycoplasma growth, a retest using double the original sample volume should be performed. If no mycoplasma is detected upon retesting, the sample is considered compliant. If mycoplasma growth persists, the sample is considered non-compliant.
Indicator Cell Culture Method
In the indicator cell culture method, the test sample is inoculated onto indicator cells (e.g., Vero cells or other cell lines approved by regulatory agencies) and cultured. After incubation, the cells are stained with a specific fluorescent dye. If mycoplasma contamination is present, mycoplasma DNA will be visible on the surface of the cells under fluorescence microscopy.
Result Interpretation: If the test result is negative, indicating no mycoplasma contamination, the sample is deemed compliant. If the result is positive or ambiguous, a retest should be conducted. If the retest is still positive, the sample is considered non-compliant.
Viral Factor Testing
For detailed procedures, refer to the article Cell Bank Characterization Guideline: Viral Factor Detection.
Tumorigenicity Testing
Tumorigenicity testing evaluates whether a cell substrate can form tumors in vivo, serving as a key characterization of the cell line's biological properties. Tumorigenicity assessments are required for newly established cell lines or novel cell substrates.
Cell Lines with Known Tumorigenicity Characteristics:
Certain passaged cell lines have been shown to be non-tumorigenic within specific passage limits, but may become tumorigenic beyond a certain passage number. For example, Vero cells require tumorigenicity testing to confirm their non-tumorigenic status within acceptable passage limits. However, once it has been demonstrated that diploid, unmodified cell lines are non-tumorigenic, such testing may not be required routinely.
Established Tumorigenic Cell Lines:
Passaged cell lines that are known to be tumorigenic (e.g., BHK21, CHO, HEK293, C127, and NS0 cells), or cell types classified as inherently tumorigenic, such as hybridoma cells, may be exempt from tumorigenicity testing when used for the production of therapeutic products.
Newly Established or Novel Cell Substrates:
For newly established or novel cell substrates with potential tumorigenic properties, quantitative methods should be employed to assess the degree of tumorigenicity. The median tumorigenic dose (TPD50) should be determined, and an evaluation of the tumorigenic risk should be conducted, taking into account the production process and the characteristics of the final product.
Testing Methods:
While in vivo testing remains the gold standard for tumorigenicity evaluation, in vitro methods, such as soft agar colony formation assays or organ culture tests, may also be employed. These methods can be particularly useful for low-passage cells or cell lines that do not exhibit tumorigenic behavior in vivo. In vitro results may serve as supplementary information for assessing tumorigenicity.
Oncogenicity Testing
Oncogenicity testing ensures that the cell substrate does not harbor factors that could immortalize the cells and induce tumor formation. The oncogenic potential of a cell substrate may be associated with the presence of oncogenic factors within the cell's DNA (or other cellular components) or within the substrate itself.
Cells of Tumor Origin or Cells with Unknown Mechanisms of Tumor Phenotype Formation:
Cells derived from tumors or those that exhibit a tumor phenotype due to unknown mechanisms are theoretically at higher risk of containing oncogenic substances.
Diploid Cell Lines with Established Histories:
Diploid cell lines with well-established histories, such as MRC-5, 2BS, KMB17, WI-38, and FRhL-2, are not typically required to undergo oncogenicity testing when used to establish a master cell bank. Similarly, passaged cell lines with extensive applications, such as CHO, NS0, Sp2/0, and low-passage Vero cells, do not require oncogenicity testing.
New Cell Substrates and Tumorigenic Phenotype:
New cell substrates, especially those with a known or suspected tumorigenic phenotype, must undergo oncogenicity testing when intended for use in vaccine production.
Testing of Cell Lysates and DNA for Oncogenicity:
Oncogenicity testing can be conducted using cell lysates and/or cell DNA. If there is a suspicion of an oncogenic virus based on the phenotype or origin of the cell substrate, it is recommended to inoculate animals with the cell lysate for oncogenicity testing. In cases where the cell substrate exhibits a tumorigenic phenotype, animal inoculation with the cell DNA should be performed for oncogenicity evaluation.
Further Investigations for Progressive Nodules:
If progressive nodules are observed during oncogenicity testing, further studies should be conducted to identify the oncogenic factors or activity. Additionally, the applicability of the cell line for its intended use must be reassessed.
Contact Us for Protein Drug Characterization Services
Cell bank characterization is a pivotal step in biopharmaceutical development, laying the groundwork for subsequent production and safety assessments. Once cell bank establishment and characterization are complete, the next challenge lies in ensuring protein therapeutics maintain their efficacy, safety, and stability throughout clinical trials and market distribution.
As specialists in protein therapeutic characterization, we at Creative Proteomics understand the critical importance of maintaining drug quality and stability throughout the development process. Our comprehensive protein therapeutic characterization services are designed to support you in the post-cell bank characterization phase:
Protein Purity and Quality Control
We employ precise analytical methods, including HPLC, SDS-PAGE, and mass spectrometry, to ensure protein therapeutics meet the highest standards of purity, content, and structure.
Structure and Function Characterization
Advanced techniques are utilized to elucidate protein tertiary structure and biological activity, providing robust assurance for subsequent production and application.
Immunogenicity Assessment
We detect potential immune responses to protein therapeutics, ensuring their safety in clinical applications.
Stability and Stress Testing
By simulating various environmental conditions, we evaluate protein stability, generating crucial data to support long-term storage and transportation strategies.
The quality of cell banks directly influences downstream production processes, while protein therapeutic characterization and monitoring are essential for clinical success and market competitiveness. For more detailed information on protein therapeutic characterization or to discuss tailored characterization solutions for your project, please don't hesitate to contact our expert team. We are committed to providing comprehensive technical support throughout your drug development journey.






