The characterization of cell banks is a critical step in the development and manufacturing of antibody and protein therapeutics, ensuring both the safety and efficacy of the final product. A fundamental aspect of this characterization process involves the thorough detection of viral factors that may pose risks to product quality or patient safety. Viral contaminations, either from endogenous or exogenous sources, can result in significant concerns, including immune reactions, unintended product alterations, or even transmission of infectious agents.
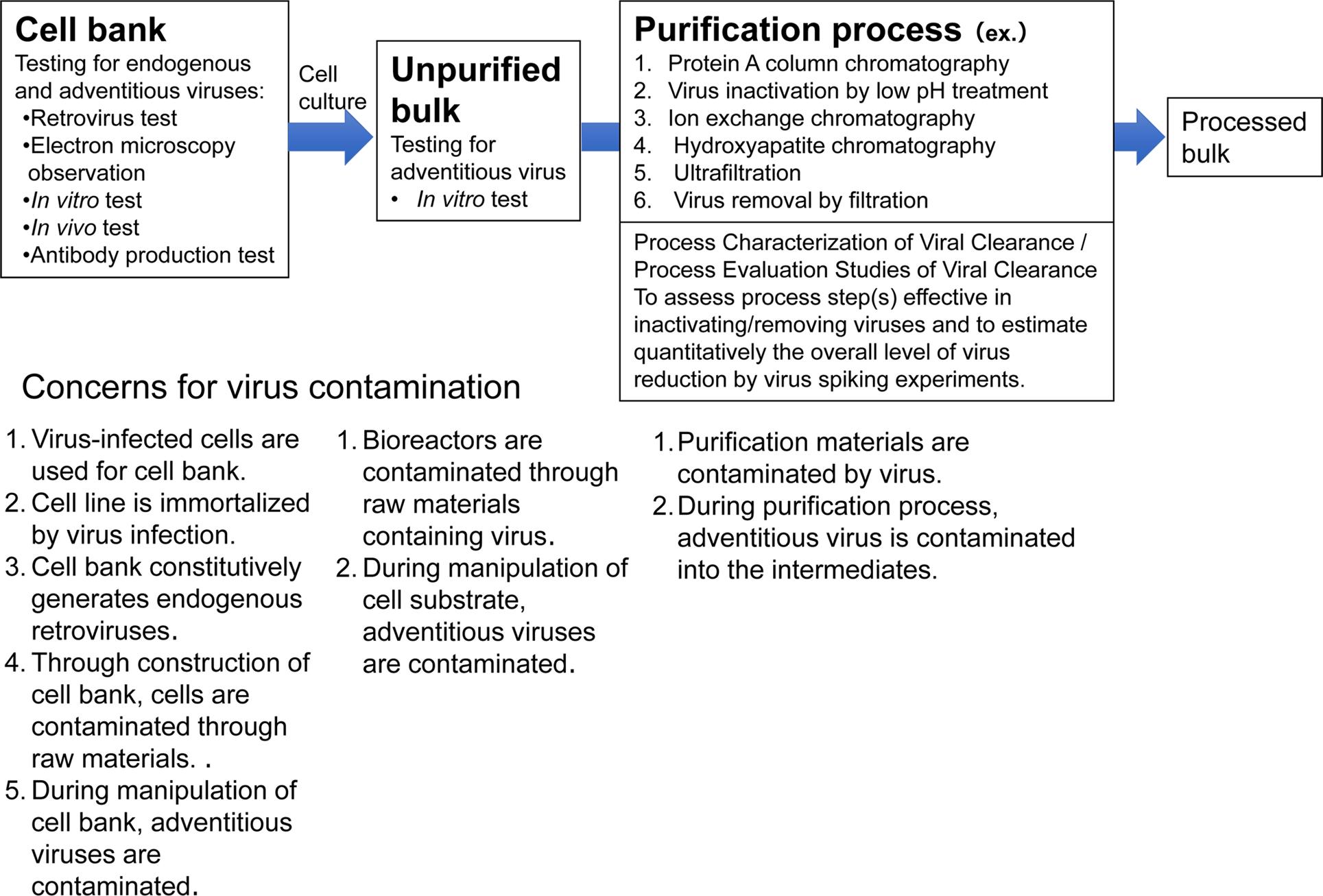 Fig. 1. Viral safety evaluation of biotechnological products derived from cell lines. (Keisuke YUSA et al,. 2020)
Fig. 1. Viral safety evaluation of biotechnological products derived from cell lines. (Keisuke YUSA et al,. 2020)The Cell Bank Characterization Guideline for viral factor detection outlines several key methods to ensure the safety and quality of cell banks used in biological product manufacturing. These methods include:
Cell Morphology Observation and Hemadsorption Assay
In this assay, inoculate a minimum of six cell culture flasks or dishes. Once the cells have formed a monolayer or reached a sufficient density, replace the culture medium with maintenance medium and continue incubation for an additional two weeks. Medium replacement can be performed as necessary. Cells should be examined daily under a microscope, ensuring that they maintain normal morphological characteristics. For adherent or semi-adherent cell lines, after a minimum of 14 days of culture, remove one-third of the cells from the culture vessels. These cells should then be subjected to a hemadsorption assay using a mixed suspension of 0.2%–0.5% guinea pig red blood cells and chicken red blood cells.
One set of cultures should be incubated with red blood cells at 2–8°C for 30 minutes, while the other set should be incubated at 20–25°C for the same duration. After incubation, both sets should be observed under a microscope to assess red blood cell adsorption. The expected result is a negative outcome, where no red blood cell adsorption is observed.
In Vitro Assay for Viral Factor Detection
To detect viral factors, prepare cell supernatants or cell lysates from the test cells. These samples should be inoculated onto at least three types of monolayer indicator cells, including monkey-derived cells, human diploid cells, and cells of the same species and tissue origin. Prior to testing, samples may be stored at temperatures of −70°C or lower.
For each type of monolayer indicator cell, inoculate at least 10^7 live cells or an equivalent quantity of cell lysate. The inoculation volume should comprise at least one-quarter of the maintenance medium. Each indicator cell type should be inoculated into at least two separate flasks. After 7 days of incubation, one flask from each cell type should be selected. The supernatant or cell lysate should be harvested and inoculated onto freshly prepared indicator cells for a second passage. The second passage should be cultured for an additional 7 days, and cell morphology should be monitored for cytopathic effects. At the end of the incubation period, collect the cell cultures for hemadsorption and red blood cell agglutination assays. The supernatant from the culture should also be tested for red blood cell agglutination.
For the hemadsorption and red blood cell agglutination assays, prepare a mixed suspension of 0.2%–0.5% guinea pig red blood cells and chicken red blood cells. Add the mixed red blood cells to the cell cultures, incubating one set at 2–8°C for 30 minutes, and the other at 20–25°C for the same duration. Subsequently, examine the cultures microscopically to observe any red blood cell adsorption. For the red blood cell agglutination assay, dilute the supernatant from the original culture serially, and then add the red blood cell suspension. Incubate first at 2–8°C for 30 minutes and then at 20–25°C for 30 minutes, observing the red blood cells for agglutination.
None of the indicator cells should exhibit cytopathic effects, and both the hemadsorption and red blood cell agglutination assays should yield negative results. A positive virus control should be included in each experiment, including a control for observable cytopathic effects, hemadsorption, and red blood cell agglutination. If the test cell lysates interfere with the monolayer cells, any potential interference must be addressed and excluded from the analysis.
If the test cells are known to support human or monkey cytomegalovirus (CMV) replication, observe the inoculated human diploid cells for a minimum of 28 days. No cytopathic effects should be observed, and both the hemadsorption and red blood cell agglutination assays should remain negative.
In Vivo Assay for Exogenous Virus Detection
To detect exogenous viral factors, prepare live cells or an appropriate equivalent of cell lysates from the test cell culture supernatants, and inoculate them into animals for in vivo viral factor testing. The test cells should be inoculated into at least four groups of animals: neonatal rats, adult mice, and chicken embryos (with two groups of different ages). For newly established cell lines, guinea pigs should also be included. For primary monkey kidney cells, herpes B virus testing should be performed using either the rabbit model or by culturing rabbit kidney cells.
If any abnormalities or disease symptoms appear in the inoculated animals, an in-depth investigation should be conducted to determine the cause. Animals that die during the observation period should undergo a thorough necropsy and histological examination to ascertain the cause of death. If the animals display symptoms indicative of viral infection, further identification of the virus should be carried out using cell culture methods or molecular biology techniques. If more than 20% of the animals die during the observation period and the deaths are clearly attributable to factors such as animal bites, the trial should be considered invalid, and the experiment must be repeated.
Criteria for Evaluation: At the end of the observation period, the following conditions must be met for the trial to be considered successful:
- Neonatal rats and adult mice inoculation groups: At least 80% of the animals must survive, and no transmissible factors or other viral infections should be observed.
- Chicken embryo inoculation group: In embryos injected into the yolk sac, at least 80% survival is required, with no evidence of viral infection. In embryos inoculated into the allantoic cavity, at least 80% survival must be maintained, and the allantoic fluid should yield negative results in the red blood cell agglutination test.
- Guinea pig inoculation group: At least 80% of the animals must survive, with no evidence of transmissible factors or viral infections.
- Rabbit inoculation group: At least 80% of the animals must survive, with no evidence of transmissible factors or other viral infections, including those related to partial injuries from the inoculation.
Detection of Retroviruses and Endogenous Viruses or Viral Nucleic Acids
Reverse Transcriptase Activity Assay: Sensitive methods, such as the product-enhanced reverse transcriptase assay (PERT or PBRT), should be employed. However, as some cellular components also exhibit reverse transcriptase activity, cells that test positive for reverse transcriptase should undergo further verification to confirm the presence of infectious retroviruses.
Transmission Electron Microscopy (TEM): At least 10^7 live cells should be processed using ultrathin sectioning for observation under a transmission electron microscope. This technique enables the identification of viral particles within the cellular structure.
Polymerase Chain Reaction (PCR) or Other Specific In Vitro Methods: When reverse transcriptase activity results are inconclusive or when reverse transcriptase assays cannot be performed, species-specific retrovirus detection methods should be employed. These include retrovirus-specific PCR, immunofluorescence, and enzyme-linked immunosorbent assays (ELISA). Quantitative PCR can also be utilized for the quantification of retrovirus particles.
Infectivity Assay: To assess the presence of infectious retroviruses, the test cells should be inoculated onto retrovirus-sensitive cell lines, followed by culturing and subsequent detection. Depending on the species origin of the test cells, different or multiple sensitive cell lines may be required for the retrovirus infectivity assay.
Principle: Different diagnostic methods possess distinct characteristics. Reverse transcriptase activity suggests the potential presence of retroviruses, while transmission electron microscopy and specific PCR assays can confirm the existence and quantify viral particles. The infectivity assay further establishes whether infectious retrovirus particles are present. Therefore, a combination of these methods should be employed for comprehensive detection. If reverse transcriptase activity is detected, it is essential to conduct transmission electron microscopy, PCR assays, and infectivity tests to confirm the presence of infectious retrovirus particles. Cells that produce infectious retrovirus particles and whose downstream processes fail to demonstrate viral clearance should not be used in production.
Detection of Species-Specific viruses
The selection of viral species for detection should be based on factors such as the cell line or strain's species origin, tissue source, and the health status of the donor. If no species-specific viruses are detected in the MCB or WCB, no further testing is required in subsequent stages.
Human-Derived Cell Lines/Strains: For human-origin cell lines, testing should consider viruses such as Epstein-Barr virus (EBV), human cytomegalovirus (HCMV), human retroviruses (HIV-1/2, HTLV-1/2), human hepatitis viruses (HAV, HBV, HCV), human parvovirus B19, human papillomavirus, human polyomavirus, difficult-to-culture human adenoviruses, and human herpesviruses 6, 7, and 8.
Rodent-Derived Cell Lines: For mouse, rat, or hamster-derived cell lines, species-specific viruses can be detected through antibody production assays (Mouse Antibody Production [MAP], Rat Antibody Production [RAP], and Hamster Antibody Production [HAP]).
Monkey-Derived Cell Lines/Strains: For monkey-derived cell lines, testing should include the detection of simian polyomavirus (e.g., SV40), simian immunodeficiency virus (SIV), and other relevant monkey-specific viruses.
Detection Methods: Appropriate in vitro techniques, such as molecular methods, should be used for the detection of these viruses. The chosen methods must demonstrate sufficient sensitivity to ensure the safety of the final product.
Bovine-Derived Virus Detection
If bovine serum was used during the establishment or passage history of the cell substrate prior to the creation of the master cell bank (MCB), working cell bank (WCB), and/or terminal production cells, these should undergo at least one detection of bovine-derived viruses. To perform the test, the cells to be examined should be prepared using culture supernatant, processed to yield a lysate equivalent to at least 10^7 viable cells/mL.
If bovine serum is no longer used in subsequent production stages, and both the MCB and/or End of Production Cell (EOPC) testing show no evidence of bovine-derived viral contamination, then this testing may be discontinued in subsequent stages of production.
Porcine-Derived Virus Detection
If porcine trypsin was used during the establishment or passage history of the cell substrate before the creation of the MCB or WCB and/or in cells exceeding production limits, testing for exogenous viruses associated with the porcine source animal, such as porcine parvovirus or bovine parvovirus, must be performed. Should porcine trypsin no longer be used in the subsequent production process, and both MCB and/or EOPC testing confirm the absence of related animal-derived viral contamination, this test may be omitted from future stages of the process.
In cases where recombinant trypsin is used, the potential introduction of exogenous viruses through the trypsin production process should be evaluated to determine the appropriate viral species and methods for detection.
Detection of Other Specific Viruses
The selection of viruses to be tested should be based on the characteristics of the cell line, its passage history, or the culture process employed. Some cell types may only be susceptible to specific viruses that cannot be detected using standard methods. In such cases, specialized detection techniques are required, such as testing for mouse parvovirus contamination in Chinese Hamster Ovary (CHO) cells.
For comprehensive cell bank characterization content, please refer to the article "Cell Bank Characterization Guideline: Technical Requirements."






