Recombinant monoclonal antibodies are central to the field of biopharmaceuticals; however, their charge heterogeneity — the subtle variations in the charge distribution between molecules within the same batch — can have profound effects on drug quality, efficacy, and safety. These charge differences primarily arise from post-translational modifications (such as deamidation and glycosylation) or process-related factors (including medium composition and pH), which lead to uneven charge distribution on the antibody surface. The presence of charge heterogeneity can alter the pharmacokinetic properties of antibodies, reduce target binding affinity, and even increase the risk of immunogenicity. Therefore, accurate characterization of charge variants and the development of strategies to control these variations are critical aspects of antibody drug development. This article provides a comprehensive analysis of the origins of charge heterogeneity, methods for its analysis, and its impact on drug properties, while exploring process control strategies from upstream production to downstream purification.
Overview of Charge Heterogeneity Characterization Methods
Isoelectric Focusing Electrophoresis (IEF)
Isoelectric focusing electrophoresis (IEF) is an essential technique for characterizing charge heterogeneity in recombinant monoclonal antibodies. This method utilizes the isoelectric point (pI) and net charge of molecules to facilitate antibody separation. Under the influence of an electric field, antibodies migrate within a medium containing ampholytes until they reach a pH matching their isoelectric point, at which their net charge is neutralized, ceasing further migration. Detection of antibodies is generally achieved by monitoring ultraviolet absorption at 280 nm.
Common IEF variants include imaging capillary isoelectric focusing electrophoresis (icIEF) and capillary isoelectric focusing electrophoresis (cIEF). Following the focusing process, icIEF does not necessitate subsequent migration, minimizing the influence of external factors and rendering it suitable for virtually all samples. A universal or slightly optimized method can yield robust analytical outcomes. Conversely, in cIEF, post-focusing migration may disrupt the focusing effect, leading to reduced resolution and prolonging the analysis time for individual samples. However, cIEF offers greater flexibility in adjusting variables during method development, making it adaptable to various electrophoresis modes using general-purpose capillary electrophoresis instruments, thus meeting diverse detection requirements.
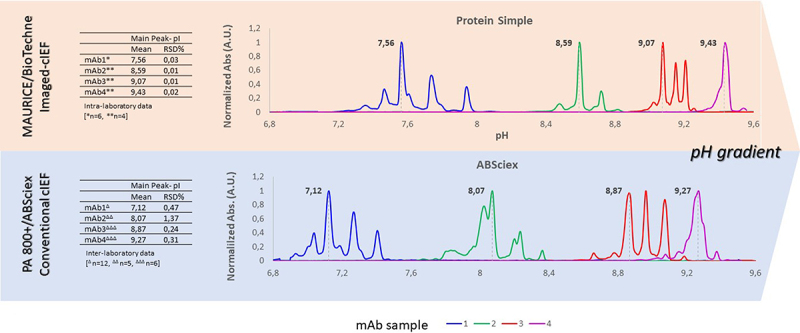 charge heterogeneity profiles by cIEF
charge heterogeneity profiles by cIEFIon-Exchange Chromatography (IEX)
Ion-exchange chromatography (IEX) represents a pivotal technique for addressing charge variants. Many therapeutic antibodies possess basic isoelectric points (pI), making cation-exchange chromatography (CEX) the most prevalent form of IEX employed. The core principle involves exploiting the differential electrostatic interactions between antibodies and ion-exchange groups on the stationary phase for separation. Antibodies with varying charges exhibit differing affinities for binding to the ion-exchange resin. By modulating the ionic strength or pH of the mobile phase, it is possible to selectively elute antibodies based on their respective charges.
Compared to other related methodologies, such as hydrophobic interaction chromatography (HIC-HPLC) and reversed-phase high-performance liquid chromatography (RP-HPLC), IEX is particularly adept at isolating charge discrepancies. HIC-HPLC primarily relies on differences in hydrophobicity for separation, while RP-HPLC is commonly utilized for analyzing small, hydrophobic molecules. In the realm of charge variant analysis, IEX offers distinct advantages, effectively segregating recombinant monoclonal antibodies into acidic, principal, and basic components. This capability is pivotal in antibody development and quality control.
Liquid Chromatography-Mass Spectrometry (LC-MS) and Enzymatic Treatment Coupled with Chromatographic Analysis
Liquid chromatography-mass spectrometry (LC-MS) along with enzymatic treatment followed by chromatographic analysis, plays a critical role in elucidating the underlying causes for the formation of charge variants. LC-MS harnessed the separation capability of liquid chromatography combined with the detection and identification prowess of mass spectrometry to enable precise molecular weight measurement and structural analysis of isolated charge variants. The data obtained from mass spectrometry facilitates the deduction of the types and sites of modifications occurring on antibody molecules, clarifying the causes behind charge variant formation. For instance, in investigating glycosylation modifications of antibodies, LC-MS can accurately determine the glycosylation sites and glycan structures.
Enzymatic treatment coupled with chromatographic analysis involves treating antibodies with specific enzymes to induce targeted modifications at certain sites, followed by chromatographic assessment of antibody alterations pre- and post-treatment. This approach allows inference of the presence and nature of modifications. For example, treating antibodies with glycosidases can elucidate the impact of glycosylation modifications on charge variants. Such methodologies provide targeted insights into the contributions of specific modifications to charge heterogeneity, thereby offering robust support for a comprehensive understanding of the mechanisms behind charge variant formation.
You may interested in
Select Service
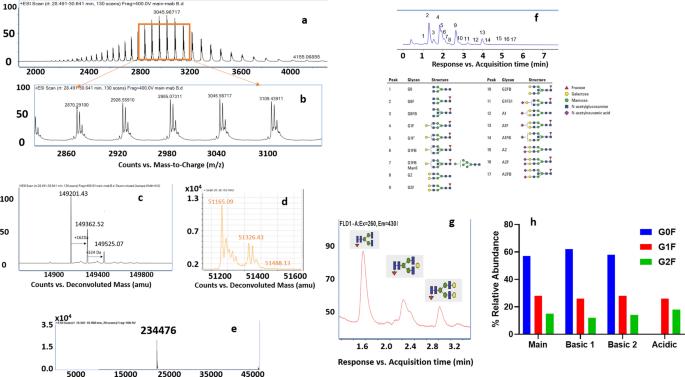 The figure illustrates the MS characterization of the bevacizumab main product, highlighting: (a) the raw MS spectrum; (b) zoomed view revealing three structural isoforms; (c-e) deconvoluted spectra indicating glycan heterogeneity with occupancy in the heavy chain but not the light chain; (f-g) glycan composition analysis using a 2-AB linked glycan library and a released glycan assay; and (h) comparison of glycosylation distribution among isolated charge variants.
The figure illustrates the MS characterization of the bevacizumab main product, highlighting: (a) the raw MS spectrum; (b) zoomed view revealing three structural isoforms; (c-e) deconvoluted spectra indicating glycan heterogeneity with occupancy in the heavy chain but not the light chain; (f-g) glycan composition analysis using a 2-AB linked glycan library and a released glycan assay; and (h) comparison of glycosylation distribution among isolated charge variants.Charge Heterogeneity-Related Protein Modifications
1. Protein Modifications Leading to Acidic Variants
| Modification Type | Mechanism | Affected Area |
| Sialic Acid Modification | Sialic acid inherently carries an anionic charge. Its modification of antibodies introduces these anions, resulting in the formation of acidic variants. | Entire antibody molecule |
| Asparagine (Asn) Deamidation | Deamidation introduces anions, predominantly occurring in the variable regions of antibodies, particularly in the complementarity-determining regions (CDRs). | Antibody variable region, specifically CDRs |
| Reaction of Reducing Sugar Aldehyde Groups with Amino Groups | Aldehyde groups on reducing sugars react with the amino groups on lysine, histidine, or arginine residues, altering charge properties. | Regions involving side chains or N-terminal related amino acids |
| Atypical Disulfide Bond Linkage | Alters the overall charge distribution within the antibody molecule. | Regions involving disulfide bond connections |
| Antibodies with Trisulfide Bonds or High Mannose Content | Influence the charge characteristics of the antibody. | Regions containing trisulfide bonds or high mannose content |
| Maleic Acid Modification at the N-termini of Heavy and Light Chains | Generates acidic variants. | N-termini of heavy and light chains |
| Cysteine104 S-Cysteamine Modification in the Heavy Chain CDR3 | Alters local charge properties. | Heavy chain CDR3 region |
| Antibodies with Reduced Disulfides, Non-reduced Components, and Cleavage Fragments | Affects the overall charge distribution of the antibody. | Regions associated with the respective structures |
2. Protein Modifications Leading to Basic Variants
| Modification Type | Mechanism | Affected Area |
| Removal of C-terminal Lysine Residues | Incomplete cleavage by carboxypeptidase B leads to the retention of positively charged lysine residues, causing basic variant formation. | C-terminal of antibodies |
| Aspartic Acid Isomerization | The formation of succinimide (Asu), a common intermediate, is critical for generating basic variants, though Asu may also produce acidic variants. | Aspartic acid residue |
| Incomplete Cyclization of N-terminal Glutamine to Pyroglutamate | Incomplete cyclization results in retention of initial glutamine, influencing charge properties. | N-terminal of antibodies |
| Methionine Oxidation | Oxidation by temperature and light affects neighboring amino acid ionization, leading to changes in charge distribution. | Methionine and neighboring residues |
| Proline Amidation after Terminal Lysine Removal | Amidation of proline after removal of C-terminal lysine in antibodies with a heavy chain C-terminal proline-glycine-lysine (PGK) sequence contributes to basic variant formation. | Heavy chain C-terminal PGK sequence |
These modifications influence the charge properties of antibodies through various mechanisms, leading to either acidic or basic variants, depending on the specific modification and affected region.
Impact of Charge Variants on Product Characteristics
1. Effects on Safety and Efficacy
| Impact Factor | Acidic Variants | Basic Variants |
| In Vivo Retention Time | Reduced | Generally unaffected; in some cases, positive effects observed |
| Blood Clearance Rate | Accelerated | Generally unaffected; in some cases, positive effects observed |
| Efficacy | Adverse, as acidic variants of trastuzumab exhibit reduced binding affinity to HER2 | Generally beneficial, with basic variants often showing enhanced affinity and ADCC effects |
The data presented in the table clearly indicate the differential effects of acidic and basic variants on monoclonal antibody (mAb) drugs. For instance, acidic variants of trastuzumab exhibit a diminished binding capacity to HER2, directly compromising therapeutic efficacy. Conversely, basic variants typically have a positive impact on drug efficacy, often enhancing both antibody-antigen affinity and antibody-dependent cellular cytotoxicity (ADCC). However, when the isoelectric point (pI) difference between variants exceeds 1 unit, significant disparities in tissue distribution and pharmacokinetics can occur, potentially influencing both safety and efficacy. Therefore, controlling charge variants is critical during the development and production of monoclonal antibodies.
2. Potential Effects on Antibody Structure and Function
The impact of modifications on antibody structure, stability, and biological function varies according to the location of the modification. Modifications at the N-terminus or C-terminus, such as the removal of lysine residues or the cyclization of glutamine to pyroglutamate, typically do not substantively alter antibody structure or stability. Additionally, these modifications do not affect complement-dependent cytotoxicity (CDC) or ADCC, as these regions are exposed and are not involved in ligand binding.
In contrast, modifications outside the N- and C-terminal regions can significantly affect antibody function and stability. For example, deamidation of asparagine residues in the complementarity-determining regions (CDRs) can reduce antibody-antigen binding affinity, potentially disrupting the ability of the antibody to bind its target or decreasing its binding strength. Deamidation-induced isomerization to isoaspartic acid can also destabilize hydrogen bonds within the CDR region, reducing its flexibility. Furthermore, modification of Cys104 in the heavy chain CDR3 by cysteamine results in a loss of thermal stability, increasing the likelihood of antibody aggregation. These findings underscore the importance of monitoring non-terminal modifications during antibody development, as they can significantly influence antibody function and stability.
Strategies for Regulating Charge Heterogeneity
Upstream Regulatory Strategies
During the upstream stages of antibody development, various strategies can be employed to regulate charge heterogeneity. One key approach involves genomics techniques, which adjust enzyme activity to modulate sialic acid modification, thereby controlling the product's charge. In addition, studying carboxypeptidase activity allows for regulation of C-terminal amino acid residue deletion, enabling control over basic charge alterations in antibodies.
Control over the culture processes and media composition is equally crucial. Numerous parameters influence basic peak formation, with pH being a significant factor. An increase in pH markedly accelerates the rate of asparagine deamidation. Temperature, too, can significantly reduce acidic peaks, likely by decreasing the transcription levels of carboxypeptidase B, thus increasing lysine variants and reducing acidic entities. Regarding culture media components, an increase in copper ion concentration and a decrease in zinc ion concentration in chemically defined media can result in an increase of C-terminal lysine variants. Raising the levels of glycerol, sodium chloride, and metal ions enhances deamidation in antibodies. Additionally, while sodium butyrate can increase antibody expression, it also elevates charge heterogeneity, particularly in basic variants. Bioflavonoids can reduce the presence of acidic variants, whereas thiosulfate may react with antibodies to form acidic entities. These strategies present effective means of controlling charge heterogeneity at the source.
Downstream Regulatory Strategies
In the downstream stages, purification techniques are primarily employed to separate charge variants. Ion-exchange chromatography (IEX) is the preferred technology, with separation methods centered on optimizing resin selection and elution strategies. The design of the stationary phase must adhere to industry standards, and chromatographic columns should be chosen based on the target protein's load and binding capacity. Membrane chromatography, though traditionally having lower binding capacity, is utilized for separating charge variants, aggregates, and host cell proteins. Enhancing its binding capacity could broaden its application potential.
Mixed-mode chromatography operates efficiently under conditions of high salt concentration, high load, a wide operational window, and high selectivity, allowing separation of charge variant impurities into distinct peaks. However, its complexity in optimization, high cost, and primary focus on aggregate issues make it generally a secondary option.
The design of elution modes offers flexibility, with common practices including salt concentration gradients and pH gradient elutions to regulate charge heterogeneity. Salt gradient elution involves charged ions from the salt competing with bound proteins for resin functional groups. pH gradient elution relies on ion strength and protein isoelectric point (pI), operating above the pI in anion exchange (AEX) mode and below the pI in cation exchange (CEX) mode. Industrial operations can achieve stepwise salt or pH gradients, though they may increase operational timeframes and costs. Presently, the ability to separate charge variants through purification means is limited by production scale capacity. A deeper understanding of protein-binding and buffer interactions, coupled with model establishment, will aid in improving downstream control of charge alterations.
Causes of Charge Heterogeneity in Antibody Production
During recombinant antibody expression, various post-translational modifications (PTMs) can lead to the formation of charge heterogeneity. Ion-exchange chromatography is commonly employed to separate antibodies into three main peak groups. Using cation-exchange chromatography as an example, the middle peak represents the primary species, with acidic variants eluting before the main peak and basic variants eluting after.
- Acidic variants typically arise from sialylation, deamidation, glycation, or antibody fragmentation. For monoclonal antibodies expressed in SP2/0 or NS0 cells, sialylation is the predominant modification contributing to acidic variants.
- Basic variants primarily result from the heterogeneity of C-terminal lysine residues, oxidation, isomerization, and succinimide formation. In the case of antibodies, the heterogeneity at the C-terminus due to incomplete removal of lysine residues is the primary cause of basic charge variants.
The presence of charge heterogeneity in antibodies can significantly impact their stability, efficacy, immunogenicity, and pharmacokinetics. Thus, monitoring surface charge heterogeneity is an essential quality control parameter in the development of antibody-based therapeutics.
Deamidation of Asparagine (Asn) and Isomerization of Aspartic Acid (Asp) are major contributors to antibody degradation, directly influencing antibody stability, in vitro bioactivity, and bioavailability. Deamidation of Asn leads to the formation of succinimide, which subsequently hydrolyzes to produce a mixture of Asp and isoAsp. Both the variable region and, in particular, the complementarity-determining regions (CDRs) of the antibody can undergo deamidation during therapeutic antibody production and storage. Deamidation in the CDRs reduces the antibody-antigen binding affinity, potentially impairing therapeutic efficacy. It has been shown that the C-terminal Fc region contains a conserved deamidation site at Asn384. Given that this residue is located in the CH3 domain and far from the Fc receptor binding sites, deamidation at this site does not appear to alter the antibody's effector function or half-life.
Glycation, a modification where glucose or lactose covalently binds to lysine or N-terminal amine groups on antibodies, is another process that can impact charge heterogeneity. Studies have shown that high glucose concentrations during cell culture may increase the degree of glycation. While glycation can influence protein biological activity and pharmacokinetic parameters, it typically does not present a significant quality concern, as it occurs uniformly across the antibody molecule and lacks distinct glycation sites. Additionally, studies confirm that glycation in the CDRs does not affect the antibody's ability to bind its antigen. For example, highly glycated IgG1 or IgG2 antibodies retain comparable affinity for Fc receptors, such as FcγRIIIa and FcRn, when compared to their non-glycated counterparts. Given the widespread presence of glucose in human serum, most plasma proteins exhibit 10-20% glycation, and thus, this modification generally does not impact immunogenicity or safety.
C-terminal Lysine Heterogeneity often results from incomplete removal of C-terminal lysine residues by carboxypeptidase B (CBP) during recombinant antibody production. The incomplete cleavage leads to multiple isoforms, including zero, one, or two lysine residues at the C-terminus. This C-terminal lysine heterogeneity is a primary contributor to the formation of basic charge variants. Comparative chromatography analysis before and after CBP digestion can clarify the proportion of basic components due to lysine removal. Research has shown that C-terminal lysine removal does not affect the antibody's antigen-binding affinity, Fc-mediated effector functions, half-life, or other biological activities. Additionally, differential scanning calorimetry (DSC) studies have demonstrated that the absence of C-terminal lysine does not affect the thermodynamic stability of IgG1 antibodies.
N-terminal Glutamine Cyclization is another common modification, where N-terminal glutamine residues in the antibody light and/or heavy chains may undergo incomplete cyclization to form pyroglutamate. Since the N- and C-termini are not involved in binding to antigens or effector function receptors, modifications at these regions generally do not affect antibody structure, stability, or activity. Studies have indicated that antibodies with one or two N-terminal glutamine residues exhibit no significant differences in biological activity.
Oxidation is a prevalent post-translational modification, commonly occurring at methionine and tryptophan residues. Methionine oxidation can occur during cell culture, purification, formulation, and storage, and is a primary cause of degradation in therapeutic antibodies in solution. The Fc region of human IgG1 contains two highly conserved methionine residues, M252 in the CH2 domain and M428 in the CH3 domain. Both residues are located near multiple ligand binding sites, and oxidation of these methionines leads to a notable decrease in antibody binding to protein A, protein G, and FcRn, as well as a slight reduction in affinity for FcγRIIa. Additionally, studies by Wang et al. have shown that methionine oxidation leads to a significant reduction in the in vivo half-life of antibodies. Some reports also suggest that oxidation may induce aggregate formation, potentially increasing immunogenicity.
References
- Vlasak, J., & Ionescu, R. (2008) Heterogeneity of monoclonal antibodies revealed by charge-sensitive methods. Journal: Current Pharmaceutical Biotechnology PMID: 18393862
- Gupta S, Jiskoot W, Schöneich C, Rathore AS. Oxidation and Deamidation of Monoclonal Antibody Products: Potential Impact on Stability, Biological Activity, and Efficacy. J Pharm Sci. 2022 Apr;111(4):903-918. DOI: 10.1016/j.xphs.2021.11.024. Epub 2021 Dec 7. PMID: 34890632.
- Oxidation and Deamidation of Monoclonal Antibody Products: Potential Impact on Stability, Biological Activity, and Efficacy. Gupta, Surbhi et al. Journal of Pharmaceutical Sciences, Volume 111, Issue 4, 903 - 918. https://linkinghub.elsevier.com/retrieve/pii/S0022-3549(21)00646-8
- Singh, S.K., Kumar, D., Malani, H. et al. LC–MS based case-by-case analysis of the impact of acidic and basic charge variants of bevacizumab on stability and biological activity. Sci Rep 11, 2487 (2021). https://doi.org/10.1038/s41598-020-79541-2









