Proteins serve as the fundamental building blocks of life, constituting essential organic molecules and acting as the primary executors of cellular functions. Life, in its absence, would not exist. Amino acids, on the other hand, serve as the basic structural units of proteins.
What are Amino Acids?
Amino acids are the fundamental units constituting the structure of proteins. In nature, there are over 300 types of amino acids, but the human body utilizes 20 specific amino acids to form proteins. These can be regarded as compounds where the hydrogen atom on the α-carbon of a carboxyl group is replaced by an amino group, all falling under the category of α-amino acids. In other words, both the amino and carboxyl groups are attached to the α-carbon atom.
 Amino acid structure formula (Gabriel Mustatea et al,. 2019)
Amino acid structure formula (Gabriel Mustatea et al,. 2019)The various amino acids share common structural features: All amino acids constituting proteins are α-amino acids. The diversity in the R side chain distinguishes different α-amino acids, serving as the basis for amino acid classification. This variability significantly influences the spatial structure and physicochemical properties of proteins. With the exception of glycine, where the R side chain is a hydrogen atom, the α-carbon atom of each amino acid is connected to four non-identical atoms or groups, making it an asymmetric carbon atom. Cysteine, among the 20 amino acids, contains a thiol group, classifying it as a thiol-containing amino acid. Methionine is predominantly present in protein molecules in the form of cysteine.
What are Peptides
Peptides are compounds formed by the linkage of amino acids through peptide bonds. A peptide bond, also known as an amide bond, is a covalent bond (-CO—NH-) formed by the dehydration condensation of the α-carboxyl group of one amino acid with the α-amino group of another amino acid. In protein molecules, amino acids are interconnected through these peptide bonds. The peptide bond is a crucial structural element that contributes to the formation of the polypeptide chain in proteins.
After the formation of a peptide chain from amino acids, the resulting entities are no longer complete amino acids, as some functional groups participate in the creation of peptide bonds. Therefore, each constituent of the protein peptide chain is referred to as an amino acid residue. Each amino acid residue possesses an R side chain, with diverse properties and functions associated with different R side chains.
Amino acid residues with a free α-amino group are designated as the amino terminus (N-terminus) and are typically positioned at the left end of the peptide chain. Conversely, those with a free α-carboxyl group are referred to as the carboxyl terminus (C-terminus) and are commonly located at the right end of the peptide chain. The direction of a polypeptide chain is designated from the N-terminus to the C-terminus, and the naming of the peptide chain follows the sequence of amino acid residues from the N-terminus to the C-terminus.
What is the Three-Dimensional Structure of Proteins?
Proteins are covalent polypeptide chains, assembled through the sequential linkage of amino acids. Every naturally occurring protein showcases a distinctive spatial configuration, commonly known as its three-dimensional structure or conformation. This intricate spatial arrangement is crucial for the overall architectural integrity of the protein. The conceptual breakdown of protein molecular structure includes primary, secondary, tertiary, and quaternary levels. While the primary structure is defined by covalent bonds, the secondary, tertiary, and quaternary structures entail spatial organizations predominantly upheld by non-covalent bonds, often referred to as secondary interactions.
 (Martin A Schroer 2011)
(Martin A Schroer 2011)1. Primary Structure of Proteins
The primary structure, alternatively termed the primary or chemical structure, pertains to the sequential arrangement of diverse amino acids within a protein molecule, linked by peptide bonds. Each protein boasts a distinct and accurate sequence of amino acids in its primary structure. Predominantly, the primary chemical bonds in this structure are peptide bonds, while certain proteins may additionally incorporate disulfide bonds (-S-S-). Owing to the robust nature of covalent bonds, the primary structure of proteins demonstrates a considerable degree of stability.
The elements encompassing: the count of polypeptide chains comprising the protein; the arrangement of amino acids within each polypeptide chain; the number and positions of intra- and inter-chain disulfide bonds. Of these factors, the most pivotal is the sequence of amino acids in the polypeptide chain. This sequence establishes the groundwork for the biological functionality of proteins, with the primary structure laying the fundamental groundwork for the exploration of more complex protein structures.
Select Service
2. Secondary Structure of Proteins
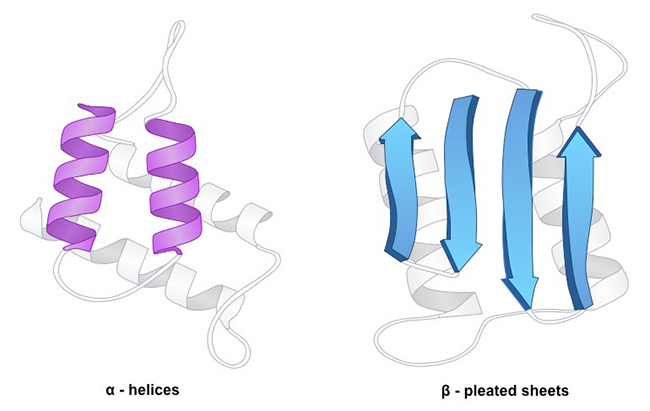
The secondary structure of proteins refers to the folding pattern of the polypeptide chain within the protein molecule. The foundation of protein secondary structure is the peptide units or the peptide bond plane, representing the local spatial arrangement of main-chain atoms, i.e., the conformation, excluding considerations of side-chain conformations. The stability of protein secondary structure is primarily maintained by hydrogen bonds.
The structural units defining the main-chain conformation include α-helices, β-sheets, β-turns, and random coils. These elements play a crucial role in shaping the overall three-dimensional architecture of proteins, contributing to their stability and biological functions.
 (Gerald Litwack, 2018)
(Gerald Litwack, 2018)Supersecondary Structure and Protein Domains:
Supersecondary structure denotes the spatial organization where neighboring secondary structures within the sequence of a polypeptide chain tend to closely associate in the folded state, establishing interactions that result in systematic aggregations of secondary structures. Presently, three foundational types of supersecondary structures have been recognized: α-helix bundle (αα); β-strand bundle (βββ); and α-helix-β-strand bundle (βαβ), with the βαβ combination prevailing as the most common.
In the realm of protein structure, domains signify an intermediate tier between secondary and tertiary structures. In more extensive protein molecules, the intimate connection of adjacent supersecondary structures along the polypeptide chain gives rise to two or more distinct domains. These domains manifest conspicuous deviations in their spatial arrangement compared to the remaining protein subunits.
Typically constituted of around 100-200 amino acid residues each, every domain boasts a distinctive spatial conformation and undertakes specific biological functions. These domains wield significant influence in delineating the comprehensive architecture of proteins, thereby contributing to their multifaceted functional diversity.
The three-dimensional spatial structure of proteins is intricately linked to their functions, and supersecondary structures serve as the fundamental units constituting these three-dimensional architectures. Predicting the three-dimensional spatial structure directly from the primary structure of a protein is a challenging task. In this context, protein secondary structures and supersecondary structures act as crucial bridges between the primary structure and the three-dimensional arrangement. Consequently, the prediction of supersecondary structures holds significant research importance as it facilitates the understanding of the complex relationship between protein sequence and function.
Select Service
3. Tertiary Structure of Proteins
The polypeptide chain of a protein, further coiling or folding based on various secondary structures, forms a well-defined three-dimensional spatial arrangement known as the tertiary structure. Some researchers also argue that the protein's tertiary structure involves the folded and coiled conformation of the main chain, along with specific conformations adopted by the side chains, contributing to the formation of distinct microenvironments, often referred to as structural domains.
The stability of protein tertiary structure primarily relies on secondary bonds, encompassing hydrogen bonds, hydrophobic interactions, salt bridges, and van der Waals forces. These secondary bonds can exist between the R groups of amino acid residues that are far apart in the primary structure. Therefore, the tertiary structure of proteins primarily refers to the interactions between the side chains of amino acid residues.
Select Service
Learn more
4. Quaternary Structure of Proteins
Proteins composed of two or more independent polypeptide chains, each with its distinct tertiary structure, form a spatial arrangement known as the quaternary structure. In this structure, the individual polypeptide chains interact through secondary bonds to create an integrated three-dimensional architecture.
Each independent unit with its unique tertiary structure within these polypeptide chains is termed a subunit. Subunits are the constituent parts participating in the formation of the quaternary structure, and each subunit corresponds to a polypeptide chain with its individual tertiary structure. The quaternary structure essentially involves the spatial arrangement, interactions, and contact points between these subunits. Although subunits possess secondary and tertiary structures independently, they lack biological activity when isolated. The complete quaternary structure is vital for the protein to exhibit biological activity.
Maintaining the quaternary structure of proteins involves non-covalent bonds such as hydrogen bonds, salt bridges, van der Waals forces, and hydrophobic interactions.
 (Gerald Litwack, 2018)
(Gerald Litwack, 2018)