The strategic design and execution of Phase II clinical trials are pivotal to the overall drug development process. By offering preliminary insights into the drug's efficacy while maintaining a focus on patient safety, Phase II trials help guide decisions regarding the continuation or cessation of drug development, thus optimizing the allocation of resources for promising new therapeutics.
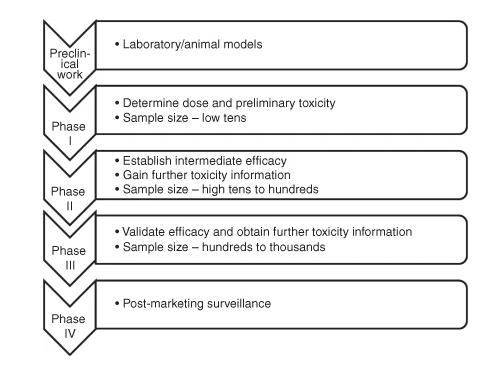 Phases of drug development.
Phases of drug development.Objectives of Phase II Clinical Trials
Upon the completion of Phase I clinical trials, where the tolerability, safety, and pharmacokinetics (PK) of a drug in humans have been established, as well as the Recommended Phase II Dose (RP2D), the initiation of Phase II clinical trials can commence. Phase II trials, also known as exploratory clinical trials, represent the first administration of the investigational drug in patient populations, with the primary objective of exploring the drug's efficacy. Given the high cost and extended duration associated with clinical research, the design of Phase II trials is critical, serving as a bridge between the preliminary safety assessments of Phase I and the more comprehensive investigations of Phase III.
The goal of Phase II trials is to strike a balance between quickly identifying promising new drugs and avoiding the premature termination of potentially efficacious therapies, while simultaneously ensuring that ineffective treatments are promptly discontinued.
Transition from Phase I to Phase II
Whereas Phase I trials emphasize safety and pharmacokinetics, Phase II trials shift the focus towards pharmacodynamics (PD) and the drug's therapeutic effects. The primary aim of Phase II trials is to preliminarily assess whether the drug demonstrates consistent pharmacodynamic effects in the target patient population while maintaining an acceptable safety and toxicity profile.
Primary Goals of Phase II Clinical Trials
Efficacy and Safety Evaluation
The main objective of Phase II clinical trials is to evaluate the initial efficacy and safety of the drug in the target patient group. Specifically, the trial seeks to determine whether the drug can achieve pharmacodynamic outcomes consistent with its intended therapeutic use, while any toxic effects remain within acceptable limits.
Exploring Key Questions
Phase II trials aim to address several critical questions regarding the investigational drug, including:
Efficacy within the RP2D: How effective is the drug for a particular indication within the dosage range confirmed as safe during Phase I trials?
Adverse Reactions and Short-term Risks: What are the short-term adverse reactions and risks associated with the drug in the target patient population?
These evaluations, focused on both efficacy and safety, lay the groundwork for deciding whether a drug should proceed to more extensive Phase III clinical trials, where its therapeutic potential can be rigorously validated on a larger scale.
Content of Phase II Clinical Trials
Trial Sequence
Phase II clinical trials typically follow a sequential approach, progressing through two distinct subphases: Phase IIa and Phase IIb. This structure allows for a systematic investigation into the drug's preliminary efficacy and safety.
Design Principles
As an exploratory stage of drug development, Phase II clinical trials may employ a variety of design strategies, including but not limited to concurrent controls, self-controls, open-label trials, three-arm trials (involving an active comparator, placebo, and the investigational drug), and dose-response studies. The primary objective of these varied designs is to comprehensively evaluate the drug's efficacy and safety in the target population.
Participants
Participants in Phase II clinical trials are recruited from patient populations that match the target indication for which the drug is being developed. These patients provide essential data on the drug's therapeutic potential in real-world clinical scenarios.
Sample Size
The sample size for Phase II clinical trials typically ranges from several dozen to several hundred participants. The exact number is determined based on the trial design, the nature of the disease, and the statistical requirements necessary to draw meaningful conclusions.
Endpoints
The key endpoints for evaluating the efficacy of a drug in Phase II clinical trials often include measures such as the Objective Response Rate (ORR), which reflects the proportion of patients exhibiting a significant reduction in tumor size. This endpoint provides an early indication of the drug's therapeutic effect.
Design Considerations for Phase II Clinical Trials
Given the exploratory nature of Phase II clinical trials, which aim to assess preliminary efficacy and safety rather than provide definitive confirmation, there is considerable flexibility in the design of these studies. For instance, in oncology trials, where spontaneous tumor regression is exceedingly rare, it can be reasonably inferred that a reduction in tumor size is attributable to the therapeutic action of the investigational drug. Therefore, a single-arm design may be employed to evaluate the drug's effect in the absence of a control group, or a dose-comparison study may be used to optimize the therapeutic dose.
Additionally, Phase II trials serve another critical purpose: the early identification and exclusion of tumor types, doses, or treatment regimens that demonstrate poor efficacy or excessive toxicity. To prevent unnecessary exposure of patients to ineffective or harmful therapies, researchers must establish predefined stopping criteria. These criteria allow for the early termination of patient enrollment if the investigational drug fails to meet efficacy or safety thresholds, thereby safeguarding patient welfare and optimizing resource allocation.
Efficacy and Safety Assessment in Phase II Clinical Trials
The Objective Response Rate (ORR) is a commonly used efficacy endpoint in Phase II clinical trials, particularly in oncology. ORR represents the percentage of patients who experience a significant and sustained reduction in tumor size, serving as a reliable early indicator of the drug's antitumor activity. The evaluation of ORR must adhere strictly to internationally recognized standards, such as the RECIST (Response Evaluation Criteria in Solid Tumors) guidelines. However, it is important to note that while ORR is an effective measure of drug activity, it does not necessarily correlate with actual improvements in overall survival or patient quality of life.
In terms of safety, Phase II trials continue to monitor adverse events observed in earlier Phase I trials and preclinical studies, with a particular focus on rare toxicities that may have gone undetected. Researchers must also conduct specialized assessments related to the drug's pharmacological class, ensuring a thorough examination of potential risks. The relationship between toxicity and dosage, as well as the reversibility of toxic effects upon discontinuation of the drug, must be carefully monitored. An important aspect of the evaluation process is determining whether the drug can achieve its intended therapeutic effect within a dosage range that is tolerable for patients or associated with reversible toxicity.
Select Service
Structural Characterization of Protein or Antibody Drugs in Phase II Clinical Trials
In Phase II clinical trials, the structural characterization of protein-based therapeutics, including monoclonal antibodies (mAbs) or antibody-drug conjugates (ADCs), is critical for ensuring drug efficacy and safety. These complex biologics exhibit unique pharmacokinetic and pharmacodynamic properties due to their large molecular size, intricate structures, and potential interactions with the immune system.
Key aspects of structural characterization include:
Primary Structure Analysis: Determining the amino acid sequence through methods like mass spectrometry (MS) ensures that the protein or antibody maintains its intended sequence fidelity.
Post-Translational Modifications (PTMs): PTMs, such as glycosylation or phosphorylation, are evaluated for their impact on the drug's stability, immunogenicity, and therapeutic activity. Techniques like glycan profiling and peptide mapping are commonly used.
Higher-Order Structure (HOS): The secondary, tertiary, and quaternary structures are assessed using spectroscopic techniques (e.g., circular dichroism or nuclear magnetic resonance) to ensure proper folding and functional integrity.
Aggregation State: Protein aggregation is a major concern for biologics as it can lead to reduced efficacy or increased immunogenicity. Analytical ultracentrifugation (AUC) and size-exclusion chromatography (SEC) are often employed to monitor aggregation levels.
Binding Specificity and Affinity: Surface plasmon resonance (SPR) and enzyme-linked immunosorbent assays (ELISA) are utilized to confirm the target-binding capability and affinity of antibodies, ensuring they retain their designed mechanism of action.
Stability Studies: Accelerated stability testing under various stress conditions (e.g., temperature, pH) helps to predict the long-term viability of the drug under clinical and storage conditions.
Comprehensive structural characterization during Phase II clinical trials provides crucial insights into the behavior of protein and antibody drugs in humans, guiding dose selection and identifying potential safety concerns before moving into later-stage trials.
Reference
- Brown, S., Gregory, W., Twelves, C. et al. Designing phase II trials in cancer: a systematic review and guidance. Br J Cancer 105, 194–199 (2011).











