Protein glycation is a prevalent non-enzymatic modification reaction that can significantly affect the quality, stability, and efficacy of antibody therapeutics. This reaction is initiated by the binding of reducing sugars, such as glucose, to lysine residues within proteins, leading to alterations in protein charge distribution, structure, and function. Although glycation occurs naturally, it poses challenges in the biopharmaceutical industry due to its potential to increase antibody heterogeneity, reduce biological activity, and elevate immunogenicity risks. Thus, understanding the mechanisms of glycation formation, assessing its impacts, and developing effective control strategies are critical elements in antibody drug development.
This paper begins with foundational insights into glycation, exploring its multifaceted impacts on antibody drugs, and offers practical methods for analysis and control. Glycation stands as an essential quality attribute in antibody drug development. By deeply understanding its mechanisms, optimizing production processes, and establishing precise analytical methods, researchers can effectively manage glycation levels, ensuring the stability, safety, and efficacy of antibody therapeutics.
Fundamentals of Protein Glycation
Definition of Protein Glycation
Protein glycation is a distinct chemical reaction characterized by the covalent attachment of reducing sugars such as glucose, fructose, or galactose to amino acid residues in proteins, with lysine being the primary target. This process occurs without the involvement of enzymes, differentiating it fundamentally from enzymatic glycosylation, which relies on specific enzymes and occurs at precise sites under stringent conditions. Unlike its enzymatic counterpart, non-enzymatic glycation is more random and can take place whenever proteins are exposed to an environment rich in reducing sugars.
Protein Glycation Mechanisms, Impacts, and Control Strategies
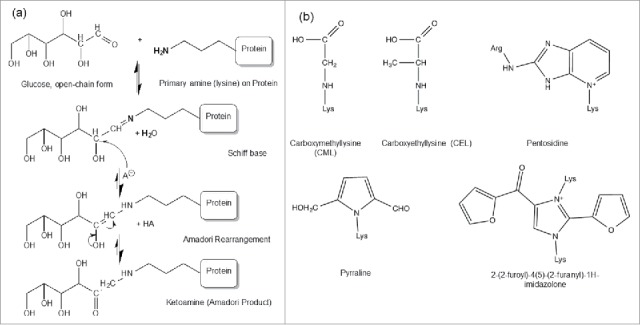 Protein glycation Maillard reactions.
Protein glycation Maillard reactions.The Impact of Protein Glycation
Protein glycation imposes a complex array of effects on proteins. Initially, it influences protein heterogeneity; the stochastic nature of glycation results in variability in both the extent and specific locations of glycation among protein molecules, thereby augmenting diversity in protein characteristics. Moreover, glycation can undermine protein stability. The structural alterations induced by glycation may increase protein susceptibility to external conditions, such as temperature fluctuations and pH changes, thereby reducing their stability. Functionally, glycation has the potential to disrupt active sites or modify the spatial configuration of proteins, adversely impacting their molecular interactions and leading to diminished or lost functional activity.
Importantly, the accumulation of advanced glycation end-products (AGEs) has been implicated in a variety of pathological conditions. In the context of diabetes, persistent hyperglycemia accelerates glycation processes, culminating in AGE accumulation and contributing to vascular complications and other health problems. Furthermore, AGEs have been observed in conditions such as osteoarthritis, the aging process, and various neurodegenerative diseases, where they play pivotal roles in disease progression.
Factors Influencing Protein Glycation
The Pivotal Role of Reducing Sugar Concentration
The concentration of reducing sugars predominantly dictates the process of protein glycation. Empirical studies have demonstrated that glycation reactions commence when glucose concentrations exceed 6 mM. Furthermore, higher glucose concentrations correlate with increased rates and extents of glycation. This phenomenon arises because elevated levels of reducing sugars provide an abundance of sugar molecules available to react with proteins, thereby accelerating the glycation process and enhancing the number of sugar molecules covalently bonded to the proteins.
Upon stabilization of glucose concentration at a particular level, the glycation process reaches a plateau. This occurs as the system achieves a dynamic equilibrium where the rate of formation of new glycation products equals the rate of decomposition or further reaction of pre-existing glycated products. Additionally, since glycation is a reversible reaction, proteins may exhibit a deglycation state when placed in a buffer with minimal or no glucose. Under such conditions, sugar moieties previously attached to the proteins may gradually detach, resulting in a reduced degree of protein glycation.
Additional Influencing Factors
Beyond the concentration of reducing sugars, parameters such as pH and temperature also significantly impact the glycation process. Variations in pH can alter the ionization states of both proteins and reducing sugars, thereby modifying their interactions and influencing the rate of the glycation reaction. Typically, an increase in temperature accelerates glycation reactions, given that higher temperatures enhance molecular activity and reaction rates. However, excessively high temperatures may lead to protein denaturation and other complications.
Protein Glycation in Antibody Production
Underlying Causes
Glycation modifications are prevalent in monoclonal antibody (mAb) products due to several underlying factors. During mAb fermentation, glucose is extensively utilized as an energy source for cellular growth and metabolism. However, this practice inadvertently facilitates glycation reactions, as glucose, being a reducing sugar, readily partakes in non-enzymatic interactions with proteins, leading to glycation modifications of the mAbs.
Moreover, the formulation stage contributes significantly to glycation. Some mAb formulations incorporate reducing sugars directly involved in glycation reactions. Additionally, commonly used sucrose can hydrolyze into reducing sugars under specific conditions, further triggering glycation. For instance, in acidic environments or at elevated temperatures, sucrose may hydrolyze, and the resulting reducing sugars can promote glycation reactions. Collectively, these factors make glycation modifications fairly common in mAb products, with potential implications for their quality and performance.
Influencing Factors
The glycation of mAbs is influenced by various factors. During fermentation, the pH of the culture medium can modify the charge properties of both proteins and reducing sugars, affecting their interactions and subsequently the glycation rate. Glucose concentration is a critical determinant; higher concentrations correlate with increased glycation rates and extents. Thus, controlling glucose levels is essential for managing glycation. Temperature also significantly influences glycation; while moderate increases can accelerate the reaction, overly high temperatures may cause protein denaturation, necessitating strict control of fermentation temperature. Furthermore, fermentation duration plays a crucial role; prolonged fermentation can increase glycation levels, requiring the determination of an optimal fermentation time to control glycation.
During storage, the formulation composition is vital. If reducing sugars are present, glycation reactions may continue to occur, thus necessitating the optimization of formulations to eliminate or minimize the use of reducing sugars. Higher storage temperatures and longer durations exacerbate glycation, impacting quality, even in typically stable lyophilized forms. Therefore, selecting appropriate storage conditions in terms of temperature and duration is crucial to mitigate the effects of glycation on mAb quality.
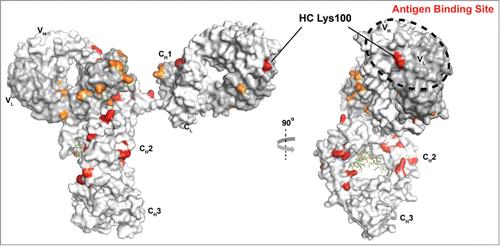 Location of glycated lysine residues in mAb1.
Location of glycated lysine residues in mAb1.Multifaceted Impacts of Glycation on Monoclonal Antibodies
Impact on Heterogeneity
Glycation markedly influences the heterogeneity of monoclonal antibodies (mAbs). This effect primarily manifests in the alteration of charge distribution, where glycation can result in the loss of positive charges at modification sites. Consequently, the overall charge heterogeneity of the mAbs shifts towards a more acidic profile. Such changes can affect the migration behavior of mAbs within an electric field, thereby influencing separation and analytical outcomes.
Moreover, glycation promotes the formation of mAb aggregates. The presence of aggregates not only impacts batch-to-batch consistency but may also affect functional activity. Variations in the degree of glycation across different batches can lead to differences in both the quantity and characteristics of aggregates, posing challenges to production stability and quality control.
Additionally, the advanced glycation end-products (AGEs) formed during later stages of glycation can alter the coloration of mAb solutions. For instance, mAbs expressed in CHO cells may exhibit a slight yellow-brown hue as a result of glycation. This change in solution color not only visually indicates the degree of glycation but may also suggest alterations in the structure and properties of the mAbs, highlighting glycation's impact on heterogeneity.
Impact on Biological Activity
The influence of glycation on antibody activity varies among different antibody molecules. Some antibodies retain their functionality post-glycation, while others may experience a complete loss of activity. This disparity largely depends on the number and spatial distribution of glycation sites.
If glycation occurs in critical regions, such as the complementarity-determining regions (CDRs) of the antibody, the binding affinity to antigens may be significantly compromised, leading to reduced activity. Since the CDRs are crucial for antigen recognition and binding, glycation can alter the structural and charge properties of these regions, disrupting the specificity and strength of antigen-antibody interactions.
Given the unpredictable effects of glycation on different mAbs, it is crucial to thoroughly assess glycation impact during the development phase. For antibodies with activity sensitive to glycation, optimizing manufacturing processes and implementing strict control measures are necessary to ensure batch-to-batch consistency in glycation levels, thereby maintaining product quality and efficacy.
Impact on Immunogenicity
Glycation has potential implications for the immunogenicity of mAbs, primarily driven by the formation of AGEs. Despite optimized production processes that minimize AGE-related glycation modifications, mAbs entering systemic circulation can undergo AGE modifications under hyperglycemic and oxidative conditions, elevating immunogenicity risk.
Antibody aggregation induced by glycation is another factor that can enhance immunogenicity. Aggregation alters the structural and physical properties of antibodies, making them more readily identified as foreign by the immune system, which can provoke an immune response.
Furthermore, AGEs can induce the expression of AGE-specific receptors on cells and promote protein cross-linking and aggregation in vivo. This non-native protein modification not only exerts cytotoxic effects on a variety of cells but may also trigger diseases, thereby exacerbating immunogenicity and posing a potential threat to the safety and efficacy of mAbs.
Methods for Analyzing Protein Glycation
Boronate Affinity Chromatography
Boronate affinity chromatography is an effective method for the separation and detection of glycation in proteins. The underlying principle involves the formation of a highly specific and reversible interaction (esterification) between tetrahedral boronate anions and the 1,2-cis-diol groups on glycated amino acids under alkaline pH conditions. In practice, non-glycated monoclonal antibodies (mAbs) do not interact with the chromatographic column and are consequently eluted directly within the mobile phase. In contrast, glycated mAbs, possessing structures capable of interacting with boronic acid, bind to the column. The application of a sorbitol step gradient then enables the elution of glycated mAbs from the column, facilitating the separation and detection of glycated versus non-glycated proteins, thereby advancing the study of protein glycation.
Using LC-MS Analysis
Liquid chromatography-mass spectrometry (LC-MS) is a powerful approach for analyzing protein glycation, focusing on both molecular weight determination and peptide mapping. From a molecular weight perspective, the occurrence of glycation at a single site results in a molecular weight increase of 162 Da. Accurate molecular weight measurements can thus be employed to ascertain glycation events and to determine the number of glycated sites. Through LC-MS analysis, precise molecular weight data are obtained and compared to theoretical values to preliminarily assess glycation status.
For the identification of specific glycated sites, peptide mapping is employed, representing an analysis of post-translational modifications. Proteins are enzymatically digested into peptides, which are then analyzed via LC-MS. Peptide maps are generated based on retention times and mass-to-charge ratios, and are compared with maps from non-glycated standards to accurately identify glycated sites. This methodology provides insights into whether glycation poses potential risks to biological activity, thereby offering robust support for in-depth studies of protein glycation.
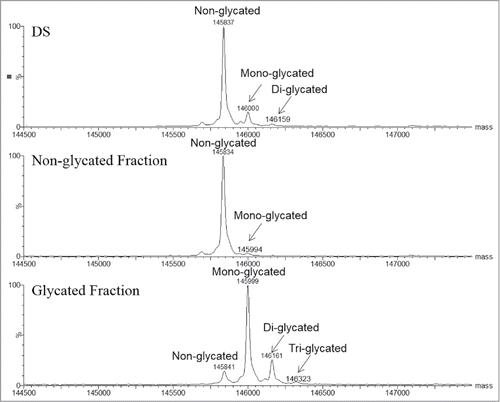 Intact LC-MS analysis of EndoS treated mAb1.
Intact LC-MS analysis of EndoS treated mAb1.Strategies for Controlling Protein Glycosylation
Characterization Studies:
During the molecular development phase, it is essential to thoroughly characterize glycosylation processes. This is particularly crucial for monoclonal antibodies (mAbs) that are susceptible to glycosylation alterations. Efforts should be made during molecular design and production process development to minimize the impact of glycosylation. A comprehensive investigation into the mechanisms, influencing factors, and specific modification sites and degrees of glycosylation on mAbs is necessary. Strict and effective control of glycosylation ratios during production ensures batch-to-batch consistency, providing a solid foundation for subsequent clinical applications and product quality assurance.
Molecular Design:
It has been observed that the proportion of acidic charge variants significantly increases in the clarified harvest fluid of multiple mAbs post-cellular fermentation, primarily due to glycosylation. This alteration in charge heterogeneity affects the stability of the mAbs production process. To mitigate glycosylation effects, molecular design can be employed. A notable strategy involves mutating lysine residues, prone to glycosylation, to arginine. This mutation is highly effective as the chemical properties of arginine differ from lysine, leading to a stable charge distribution. Importantly, this amino acid mutation does not affect the antibody's activity, effectively reducing or eliminating glycosylation modifications and ensuring production stability.
Process Control:
By establishing glycosylation kinetic models, in-depth knowledge of the relationship between glycosylation reaction rates, extents, and fermentation conditions can be obtained. This allows for precise regulation of fermentation parameters such as temperature, time, and glucose concentration, thereby effectively suppressing glycosylation while ensuring mAb quality and expression. During ion-exchange chromatography steps, glycosylated antibody components predominantly reside in the acidic charge distribution region. By adjusting the conditions appropriately, acidic components can be selectively removed, reducing the proportion of glycosylated antibodies in the final product.
For glycosylation occurring during antibody storage, evaluating and optimizing the formulation is critical. For glycosylation-prone antibodies, reducing sugars like glucose should be avoided in formulations. Screening suitable buffering salts, such as HEPES and citrate, which can lower the glycosylation rate, is pivotal. This helps minimize glycosylation reactions during storage, thereby ensuring the antibody's quality and stability.
You may interested in
Learn More
Protein Glycation and Critical Quality Attributes (CQA)
Evaluation Based on the QbD Concept:
Within the framework of Quality by Design (QbD), it is essential to assess protein glycation in antibody research and development projects, with consideration given to the specific characteristics of the antibodies. This assessment should encompass evaluations of pharmacodynamics and pharmacokinetics to holistically determine the impact of glycation on antibody efficacy, safety, and stability. If glycation is found to significantly affect the binding affinity of the antibody to its target, alter its pharmacokinetic profile, or enhance its immunogenicity, it may be designated as a Critical Quality Attribute (CQA). Conversely, if the impact is minimal, glycation may not be classified as a CQA. This evaluation methodology ensures precise and scientifically sound control over protein glycation.
Subsequent Measures:
In cases where protein glycation is identified as a CQA, stringent control measures must be implemented. During the process validation phase, a comprehensive evaluation of the production process's ability to control glycation is necessary, ensuring the stability and reliability of all parameters. The range of critical process parameters should be clearly delineated to maintain glycation levels within an acceptable range. In the commercial production phase, continual monitoring of glycation-related parameters is required, including regular assessment of glycation levels in the product. Additionally, close attention should be given to changes in raw materials, production environments, and other factors that may influence glycation levels. Through continuous monitoring and adjustment, process stability can be maintained, thereby ensuring the consistency and safety of product quality.
References
- Madhav M. Joglekar, Laxman N. Bavkar, Srinivas Sistla, Akalpita U. Arvindekar, Effective inhibition of protein glycation by combinatorial usage of limonene and aminoguanidine through differential and synergistic mechanisms,International Journal of Biological Macromolecules, 2017, https://doi.org/10.1016/j.ijbiomac.2017.02.104.
- Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10(5):339-52. DOI: 10.1016/0891-5849(91)90040-a
- Qin, Qiaojing, Jianying Niu, Zhao-xia Wang, Wangjie Xu, Zhongdong Qiao and Yong Gu. "Astragalus membranaceus Inhibits Inflammation via Phospho-P38 Mitogen-Activated Protein Kinase (MAPK) and Nuclear Factor (NF)-κB Pathways in Advanced Glycation End Product-Stimulated Macrophages." International Journal of Molecular Sciences 13 (2012): 8379 - 8387. DOI:10.3390/ijms13078379
- Wei B, Berning K, Quan C, Zhang YT. Glycation of antibodies: Modification, methods and potential effects on biological functions. MAbs. 2017 May/Jun;9(4):586-594. doi: 10.1080/19420862.2017.1300214.
- Mo, J., Jin, R., Yan, Q., Sokolowska, I., Lewis, M. J., & Hu, P. (2018). Quantitative analysis of glycation and its impact on antigen binding. mAbs, 10(3), 406–415. https://doi.org/10.1080/19420862.2018.1438796







